Self-assembling recyclable surface platforms for catalysis and sensing based on supramolecular grids
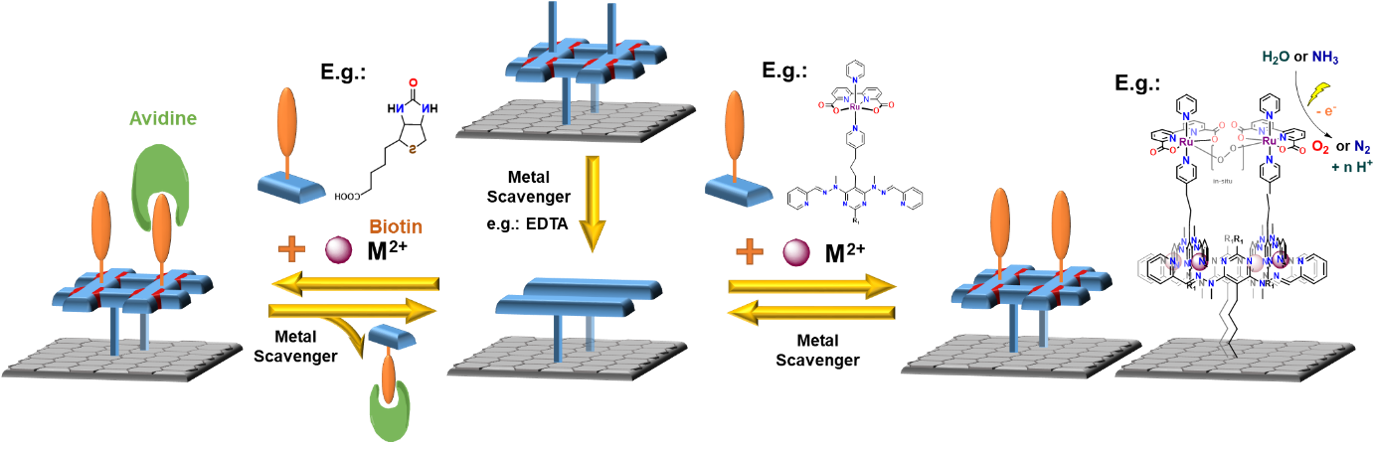
Figure 1: Assembly of surface anchored heterostranded grid and depiction of heterogenous molecular electrode with RuBda for electrochemical water oxidation
Introduction and project summary
Much effort in the field of surface engineering is devoted to the assembly of regular arrays. However, many applications do not require extensive 2D networks. Already a simple anchoring to the surface can dramatically improve different catalytic properties such as electron transfer in electrocatalysis or help in artificial sensors or scavengers for biochemistry-related projects (Biotin-based scavenging).
This project aims to prepare grid-based surface-bound platforms, which will be reversibly self-assembling/disassembling, responsive to external stimuli and customisable for different applications.
As depicted in Figure 1, anchoring a grid to a surface followed by metal removal (EDTA, cryptands) will disassemble the grid structure. This will leave behind a set of anchored ligands correctly pre-placed for a repetitive re-assembly of the grid moiety. Subsequent addition of new metal cations and readily functionalised grid ligands will lead to a new heterostranded grid formation. Due to the presence of permanently anchored ligands, it will be possible to repeat this process anytime when the functionality needs to be restored or changed, thus creating reusable/recyclable surface support for applications in different fields. (Figure 1)
Figure 1 also depicts an example of a fabrication process of a molecular electrode for heterogeneous electrocatalysis using an anchored grid functionalised with a current "state of the art" water oxidation catalysts - RuBda.[1] The catalyst is known to dimerise during the key step of its catalytic cycle. In this case, the classic individual anchoring drastically reduces its activity due to the hindrance of this process. The installation of two RuBda moieties in a well-defined (tuneable) distance within the grid scaffold should enable their dimerisation and unlock the catalysts for practical applications. The repeatable assembly will allow the recycling of the grid platform in the case of catalysts degradation, leaching or its exchange for a different catalyst.
Methodology
In the scope of this work, the students will first prepare and study the assembly and disassembly of the simple grids in solution. Afterwards, they will try to anchor the grids on different surfaces and study the assembly/disassembly process on the surface using spectroscopic and electrochemical techniques[2] (Figures 2-4).
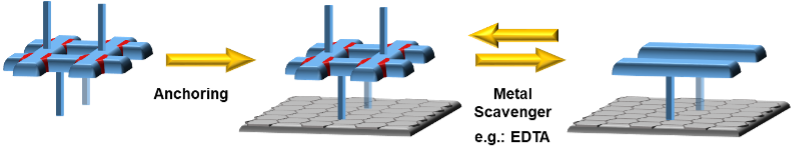
Figure 2: The proof-of-concept assembly and disassembly of the basic grid motive.
Visualisation of the whole assembly/disassembly process will also be done electrochemically using differing CV responses during the different states of assembly (Figure 3). Using different cations, one will be able to obtain different electrochemical traces and thus confirm the re-assembly of the coordination complex.
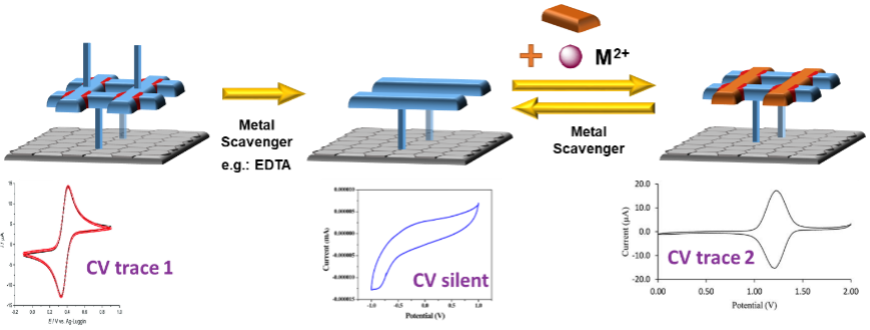
Figure 3: The use of different redox-active metals to distinguish the beginning and end states of the grid
When control over the whole process is established, we will use ligands bearing redox-active groups to prepare more complex and functional systems. In addition to using different metal cations, the ligand exchange will prove that the newly re-assembled complex contains new ligands and not only cations and will also open a path for more practical applications of the system (Figure 4).
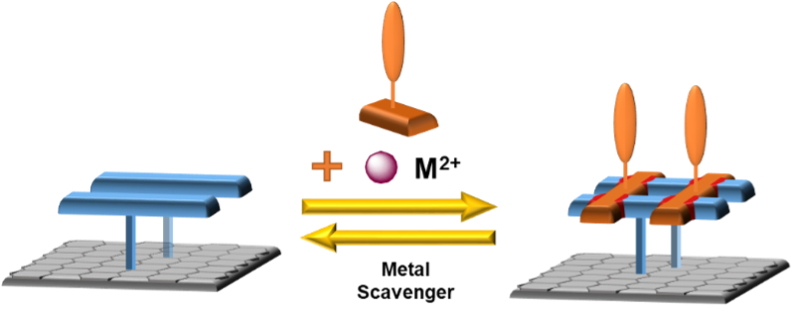
Figure 4: The use of different redox-active ligands to generate functional grid assemblies
Applications:
RuBda for electrocatalysis
In the final stage, the platform will be assembled with ligands bearing RuBda water oxidising electrocatalyst (Figure 5). The RuBda is chosen because its mechanism of function requires the formation of a (-O-O- bridged) dimer. [1] Therefore, the performance of classically covalently anchored single molecule RuBda on the surface is drastically inferior to its performance in the solution. [3] In this regard, the highly regular spatial arrangement of the grids should hold two (or more) RuBda molecules at distances favourable for dimer formation and enhance its performance in the scope of heterogenous molecular anodes in electrochemical water or ammonia oxidation catalysis. These processes are of utmost importance for our efforts to achieve a carbon-free and sustainable energy cycle.
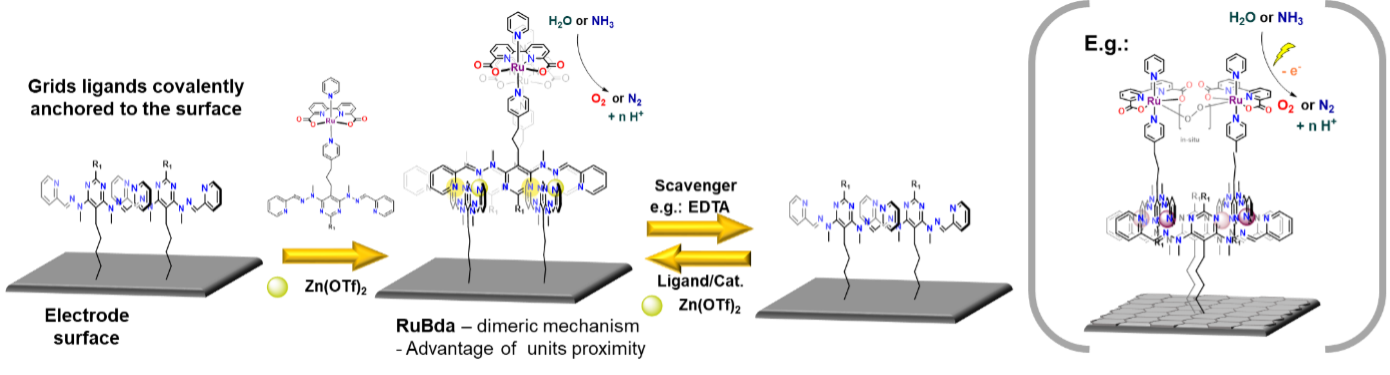
Figure 5: Functionalisation with RuBda for effective and practical (electro)catalysis
Sensors/Biochemical pull down (Far, far away future)
The same concept could also be used for biochemically relevant pull-down techniques or custom-made sensors. [4] (Figure 6)
[1] L. Duan, F. Bozoglian, S. Mandal, B. Stewart, T. Privalov, A. Llobet, L. Sun, Nat. Chem. 2012, 4, 418–423.
[2] N. Elgrishi, K. J. Rountree, B. D. McCarthy, E. S. Rountree, T. T. Eisenhart, J. L. Dempsey Journal of Chemical Education 2018, 95 (2), 197-206
[3] R. Matheu, L. Francàs, P. Chernev, M. Z. Ertem, V. Batista, M. Haumann, X. Sala, A. Llobet ACS Catalysis 2015, 5 (6), 3422-3429
[4] Mochizuki Y, Kohno F, Nishigaki K, Nemoto N. Anal Biochem, 2013, 434, 93-5
Figure 6: Biotin scavanging system used
in biochemistry for protein pulldown.
