Supramolecular grids as building blocks in responsive self-healing polymers
 Grids – building blocks for Smart Materials
Grids – building blocks for Smart Materials
In the frame of this project, new stimuli-responsive materials will be developed via the incorporation of metaloresponsive ligands and grids into the polymeric matrix.[1]
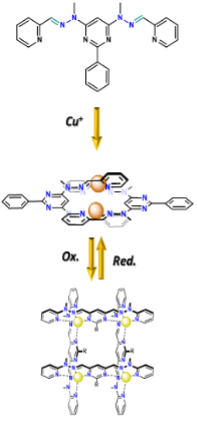
As shown in Figure 1, the grid's ligands can adopt three different states: metal-free native conformation, double helicate (Cu+, Ag+) or grid geometry (Zn2+, Fe2+, Cu2+) according to the presence and coordination preferences of metal cations (Figure 2a).[2]
Two possible strategies will be tested to incorporate ligands into the polymeric matrix. The first will be through the preparation of shorter fragments bearing the grid's ligands on their ends (Figure 2b, Left).[1] Such fragments are usually viscous liquids, which will crosslink after the addition of cations and form a solid material. The features of such a composite will depend on the type of cation and polymeric fragment. The second approach will incorporate ligands inside the polymeric chain through the co-polymerisation of peripheral groups with isocyanate ends of polymeric fragments (Figure 2b, Right).[1]
Besides the metalo-responsive behaviour, the material should also display self-healing properties, thanks to the reversible assembly of the grids and helicates (Figure 2c).
Switching between three states of the ligands will then directly affect the properties of the bulk material. These different nanoscopic changes/movements should translate into macroscopic changes of shape, density or conductivity. Furthermore, it should be possible to reversibly control these changes in a stimuli-responsive manner through the changes in the oxidation state of the metal cation (Cu+/2+). The supramolecular extension of this methodology entails applications like responsive hydrogels, artificial muscles or responsive self-assembling polymer particles.[3,1]
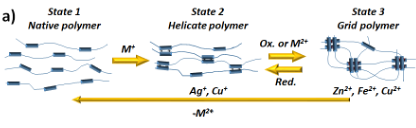
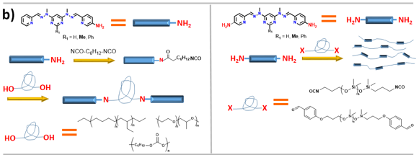
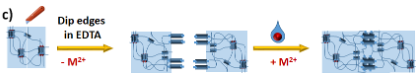
Figure 2: a) Transitions between free ligand, double helix and grid inside the polymeric matrix; b) The synthesis of polymers with incorporated ligands; c) Illustration of the self-healing process
[1] N. Roy, B. Bruchmann, J. M. Lehn, Chem. Soc. Rev. 2015, 44, 3786–3807
[2] A. M. Stadler, C. Burg, J. Ramírez, J. M. Lehn, Chem. Commun. 2013, 49, 5733–5735.
[3] J. G. Hardy, X. Y. Cao, J. Harrowfield, J. M. Lehn, New J. Chem. 2012, 36, 668–673.
