Testing the possibility of organometalic coordination complexes in preparation of bifunctional ligands modifying protein-protein interactions
Dynamic Combinatorial approach to PROTACs: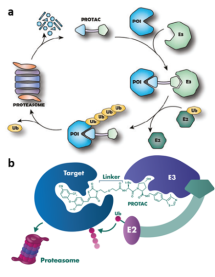
PROTACs and its mode of action
In recent years PROATACs gained much attention due to their ability to target previously "undruggable" substrates.1,2 The concept is used to label a protein of interest (PI) with ubiquitin to trigger its proteasome degradation. (Figure 1a, Top) PROTACs molecules are heterobifunctional compounds/ligands with two active parts connected through an organic linker. In a simplified way, one of the ligand's heads binds to PI intended for degradation. The other head then connects to another protein responsible for degradation. The result is a precise clearance of a particular PI, moderation of all its downstream processes and ultimately, a correction of associated pathological processes.
However, there are also certain limitations to the current PROTAC ligands. Since they must bear two, often big, binding units and an appropriately long organic linker, the size of the molecule can become a problem.5 If too big, it can lead to a lower uptake. Secondly, if the concentration of the PROTAC ligand is higher, the activity decreases due to the competitiveness and saturation of binding sites, resulting in a characteristic bell-shaped curve.6 (Figure 2, Left)
Thirdly, the covalent nature of ligands is a problem for scaffold optimisation and modification. Therefore, any change in either warheads, linker size or target PI requires lengthy synthesis of a whole ligand.
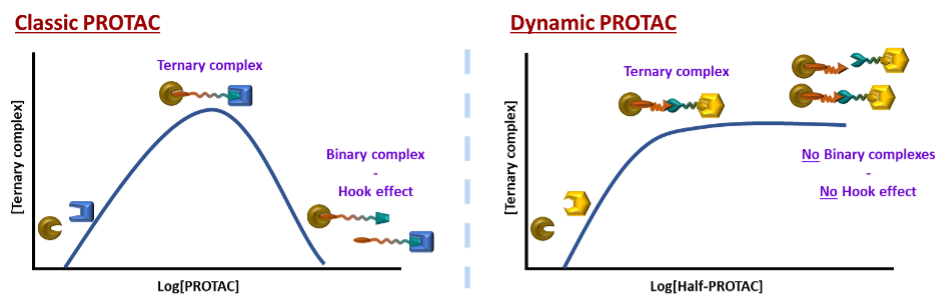
Figure 2: Left: The bell curve of the concentration dependence of Classic Protacs; Right: Foreseen improved concentration curve of dynamic PROTACs with no decrease of effectivity at higher concentrations
Dynamic combinatorial PROTACs
However, introducing a new type of dynamic linking motive could solve all the original issues. This way, the size of the delivered molecule decreases as only half-sized molecules must be delivered into a cell. The dynamic combinatorial way means a statistical formation of productive heterobifunctional specie. Therefore, since the binding events of the two heads are now independent and do not interfere with each other, there will be no hook effect, no bell-shaped concentration curve and consequently, less stringent concentration limitations. (Figures 2 Left and 3).
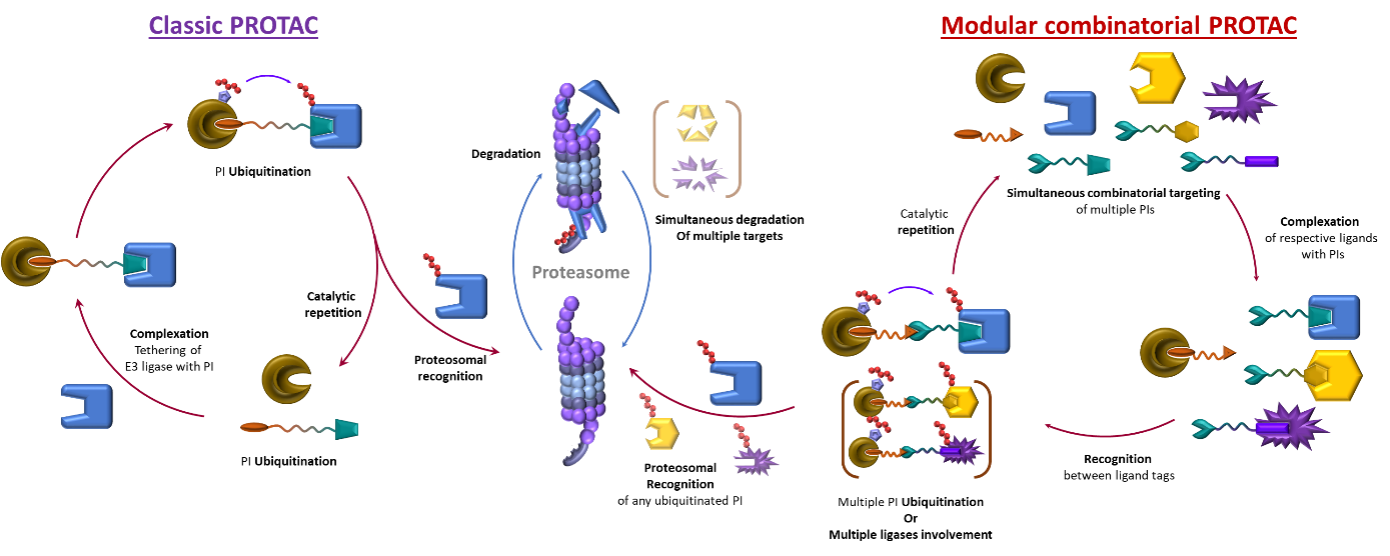
Figure 3: Left: Single target, single ligase mode of action of classic Protac; Right: Combinatorial multitargeting mode of action of Dynamic combinatorial Protac.
Using Copper as a supramolecular glue for specific and targeted PROTACs action
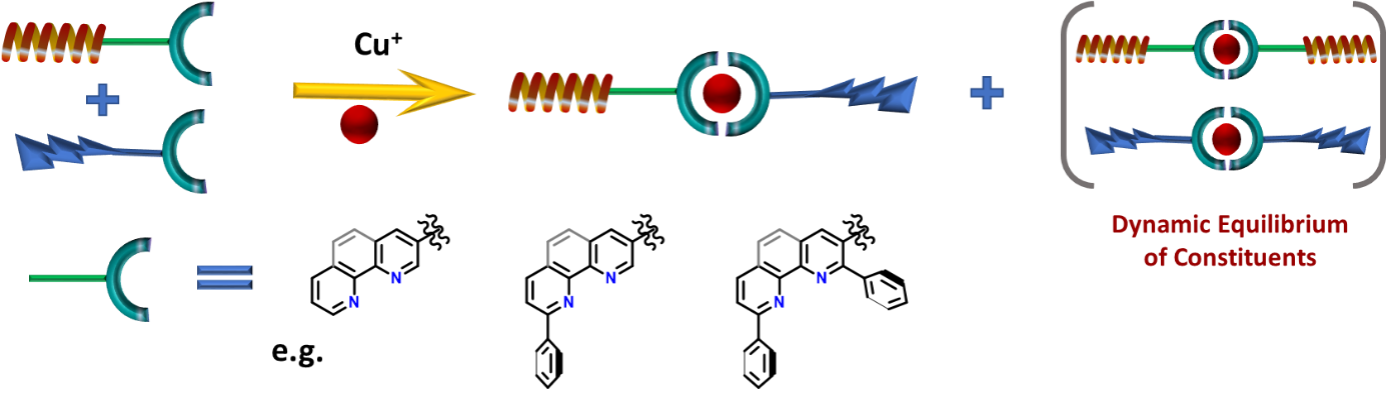
Figure 4: Illustration of the use of metal coordination as a dynamic linking motive
Advantageously, there are already known convenient, dynamic motives such as metal-ligand coordination (e.g. Copper), hydrogen bonding or other supramolecular interactions, which are suitable for this project's scope.
The primary focus will be on Copper, a biogenic metal naturally present in cells. Copper complexes are also labile, meaning they undergo rapid ligands exchange. This process is tuneable by the design of organic ligands and ensures the statistical formation of heterobifunctional active species and catalytic mode of action.7
The project will begin with synthesising model ligands based on phenanthroline and pyridines, widely used ligands with a tunable affinity towards Cu through substitution. After that, their equilibrium distribution and association constants will be studied. The best matching motives will be finally tested in cell cultures. For a proof-of-concept stage, in-vivo studies will start with fluorescent dyes instead of active moieties. This will allow us to study the distribution of ligands inside the cell and confirm their dynamic association under biological conditions. Finally, the two sets of compatible bioactive ligands will be prepared and tested for protein degradation.
The metal for proof of concept was chosen Copper for the abovementioned reasons – advantageous dynamic coordination, bioavailability and clinical relevance. However, one can easily envisage other first-row transition metals, such as iron, as another possible target.
Literature:
1) Zhong Y, Chi F, Wu H, Liu Y, Xie Z, Huang W, Shi W, Qian H. Emerging targeted protein degradation tools for innovative drug discovery: From classical PROTACs to the novel and beyond. Eur J Med Chem. 2022, 231, 114142. doi: 10.1016/j.ejmech.2022.114142
2) Qi SM, Dong J, Xu ZY, Cheng XD, Zhang WD, Qin JJ. PROTAC: An Effective Targeted Protein Degradation Strategy for Cancer Therapy. Front Pharmacol. 2021, 12, 692574. doi: 10.3389/fphar.2021.692574.
3) Li, X., Song, Y. Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy. J Hematol Oncol 13, 50 (2020). https://doi.org/10.1186/s13045-020-00885-3
4) Philipp M. Cromm, Craig M. Crews and Hilmar Weinmann, Chapter 1:PROTAC-mediated Target Degradation: A Paradigm Changer in Drug Discovery? , in Protein Degradation with New Chemical Modalities: Successful Strategies in Drug Discovery and Chemical Biology, 2020, pp. 1-13 DOI: 10.1039/9781839160691-00001
5) Cecchini C, Pannilunghi S, Tardy S, Scapozza L. From Conception to Development: Investigating PROTACs Features for Improved Cell Permeability and Successful Protein Degradation. Front Chem. 2021 Apr 20;9:672267. doi: 10.3389/fchem.2021.672267. PMID: 33959589; PMCID: PMC8093871.
6) Xin Han, Lijie Zhao, Weiguo Xiang, Chong Qin, Bukeyan Miao, Tianfeng Xu, Mi Wang, Chao-Yie Yang, Krishnapriya Chinnaswamy, Jeanne Stuckey, and Shaomeng Wang Discovery of Highly Potent and Efficient PROTAC Degraders of Androgen Receptor (AR) by Employing Weak Binding Affinity VHL E3 Ligase Ligands; Journal of Medicinal Chemistry 2019 62 (24), 11218-11231; DOI: 10.1021/acs.jmedchem.9b01393
7) Satoshi Umeki, Shoko Kume, and Hiroshi Nishihara Switching of Molecular Insertion in a Cyclic Molecule via Photo- and Thermal Isomerization Inorganic Chemistry 2011 50 (11), 4925-4933 DOI: 10.1021/ic200145h
