Screening of different coordination motives in phototriggered ligand exchange on metal complexes for targeted drug delivery
Supramolecular stapling of peptides and proteins:
Introduction
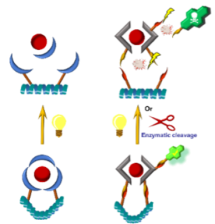
Light as a non-invasive and non-destructive force is a highly appreciated and sought way of influencing chemical reactions and physicochemical features of active compounds. It allows precise spatiotemporal focus and is cheap and selective as it influences only artificially tweaked molecules. The current development is hindered by the low penetration depth of the shortest wavelength, where most of the activity is observed.
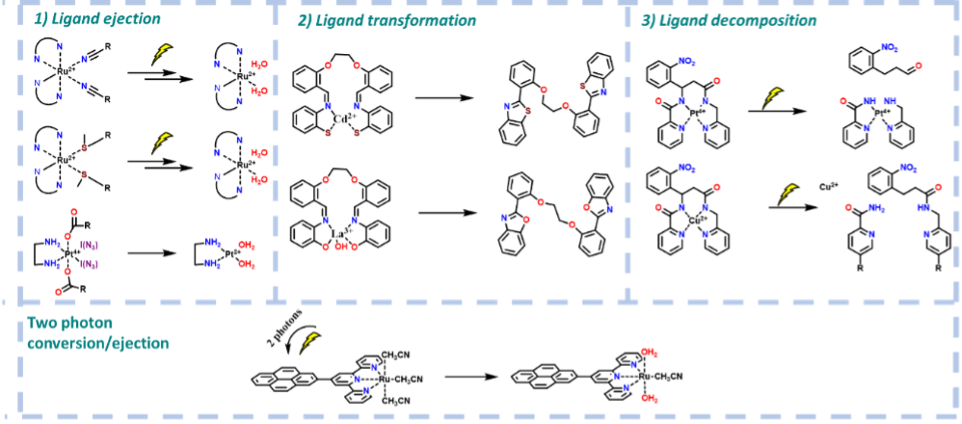
This field is nowadays dominated by pure organic compounds with photodissociating bonds. However, many photoactive complexes are also known in the literature. These are able, upon irradiation, to release their ligands and rearrange their coordination sphere. Some were already tested as vectors for drug delivery. There, the light triggered the release of the cargo only in the specific and restricted area of light impact. However, phototrigered complexes and their interactions with larger biological structures are still much undeveloped.
In comparison, the coordination compounds offer binding strength, often rivalling covalent bonds. Above that, the presence of metal often brings the interesting prospect of spatiotemporal visualisation through fluorescence or isotopic labelling. This project aims to explore ways of photo-triggered release from coordination compounds for therapeutic purposes, employing this concept in complex and unique modes of action.
The aims and methodology:
In this section, the model modes of action will describe each goal's key steps, and all will be illustrated with specific examples. The projects progressively build in complexity on Top of each other; however, underlying chemical, biological and analytical methods remain the same throughout the proposal.
Light-controlled release from Vanadium complexes
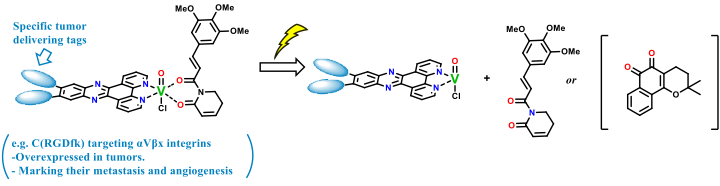
Vanadium complexes were already proven to be able to release their ligands upon light irradiation. The successful work with curcumin as a ligand showed the viability of this approach for a targeted death of tumour cells.1 One can imagine a plethora of other therapeutic compounds to be coordinated and released similarly. In order to learn the methodology and synthetic procedures, this project will start with preparing a library of Vanadium complexes with different motives, such as Piperlongumine (anticancer inhibitor) and Lapachone (ROS generation), which susceptibility towards the light will be tested.
Targeted release
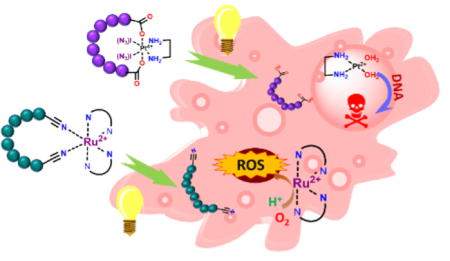 Later, when the basic methodology is mastered, more advanced topics will be studied, including short polypeptides stapled/cyclised with Ruthenium or platinum. Such complexes are relevant in various anticancer therapies. For example, Ruthenium2,3 is known to form reactive oxygen species, while platinum4 drugs are potent intercalators to DNA, both causing cell apoptosis. However, both families are highly unspecific in their cellular uptake, consequently having high off-target impact and toxicity. Connection with cell-specific delivering tags stapled together in a photouncaging complex would lead to a double layer of specificity, ensuring that only the irradiated cells are affected by the therapy. The ligand functionalisation could also be used to shift the active wavelength into a two-photon absorption range and the infrared region with considerably deeper penetration depth.
Later, when the basic methodology is mastered, more advanced topics will be studied, including short polypeptides stapled/cyclised with Ruthenium or platinum. Such complexes are relevant in various anticancer therapies. For example, Ruthenium2,3 is known to form reactive oxygen species, while platinum4 drugs are potent intercalators to DNA, both causing cell apoptosis. However, both families are highly unspecific in their cellular uptake, consequently having high off-target impact and toxicity. Connection with cell-specific delivering tags stapled together in a photouncaging complex would lead to a double layer of specificity, ensuring that only the irradiated cells are affected by the therapy. The ligand functionalisation could also be used to shift the active wavelength into a two-photon absorption range and the infrared region with considerably deeper penetration depth.
Initial in-vitro studies of photo release can be done by standard analytical techniques such as NMR, UV, and HPLC. The complexes will then be fed to cell cultures, and the IC50 of different combinations will be measured in the dark or under irradiation and analysed. The added possibility is to use isotopic labelling or fluorescent ligands to follow the journey through the organism.
Targeted delivery using accumulated metals as photosensitizers
 Furthermore, it is a well-known fact that some cancer types and diseased cells accumulate with increasing resistance certain metal cations such as Copper or Platinum. Here we would like to use the specific accumulation of platinum in cis-platinum-resistant cancer cultures. The peptidic delivering/active part will be stapled with a specific organic motive.5,6 Onto this motive, a specific active moiety will be grafted. The metal complexation will then happen directly in the affected cell and serve as a sensitisation method and activator for light-mediated release. At this stage, we will combine supramolecular chemistry with classic organic chemistry to reach cell-specific effects, hopefully obtaining the ability to differentiate between healthy and pathological cancer cells. The work will start with the incorporation of fluorescent dye to study the delivery and release in-vitro and in-vivo. Later, the same experiments will be repeated with model active compounds to achieve the desired therapeutic effect.
Furthermore, it is a well-known fact that some cancer types and diseased cells accumulate with increasing resistance certain metal cations such as Copper or Platinum. Here we would like to use the specific accumulation of platinum in cis-platinum-resistant cancer cultures. The peptidic delivering/active part will be stapled with a specific organic motive.5,6 Onto this motive, a specific active moiety will be grafted. The metal complexation will then happen directly in the affected cell and serve as a sensitisation method and activator for light-mediated release. At this stage, we will combine supramolecular chemistry with classic organic chemistry to reach cell-specific effects, hopefully obtaining the ability to differentiate between healthy and pathological cancer cells. The work will start with the incorporation of fluorescent dye to study the delivery and release in-vitro and in-vivo. Later, the same experiments will be repeated with model active compounds to achieve the desired therapeutic effect.
Literature:
1) Banerjee S, Prasad P, Hussain A, Khan I, Kondaiah P, Chakravarty AR. Remarkable photocytotoxicity of curcumin in HeLa cells in visible light and arresting its degradation on oxovanadium(IV) complex formation. Chem Commun (Camb). 2012 Aug 11;48(62):7702-4. doi: 10.1039/c2cc33576j.
2) Rapp TL, Wang Y, Delessio MA, Gau MR, Dmochowski IJ. Designing Photolabile Ruthenium Polypyridyl Crosslinkers for Hydrogel Formation and Multiplexed, Visible-light Degradation. RSC Adv. 2019;9(9):4942-4947. doi: 10.1039/C8RA09764J.
3) Qu F , Martinez K , Arcidiacono AM , Park S , Zeller M , Schmehl RH , Paul JJ , Kim Y , Papish ET Sterically demanding methoxy and methyl groups in ruthenium complexes lead to enhanced quantum yields for blue light triggered photodissociation. Dalton Trans. 2018 Nov 13;47(44):15685-15693. doi: 10.1039/c8dt03295e.
4) Stefanie Perfahl, Marta M. Natile, Heba S. Mohamad, Christiane A. Helm, Carola Schulzke, Giovanni Natile, and Patrick J. Bednarski Molecular Pharmaceutics 2016 13 (7), 2346-2362 DOI: 10.1021/acs.molpharmaceut.6b00108
5) Ciesienski, K.L., Hyman, L.M., Yang, D.T., Haas, K.L., Dickens, M.G., Holbrook, R.J. and Franz, K.J. (2010), A Photo-Caged Platinum(II) Complex That Increases Cytotoxicity upon Light Activation. Eur. J. Inorg. Chem., 2010: 2224-2228. https://doi.org/10.1002/ejic.201000098
6) Kumbhar AA, Franks AT, Butcher RJ, Franz KJ. Light uncages a copper complex to induce nonapoptotic cell death. Chemical Communications (Cambridge, England). 2013 Mar;49(24):2460-2462. DOI: 10.1039/c3cc38927h
