stdClass Object
(
[nazev] => Ústav anorganické chemie VŠCHT Praha
[adresa_url] =>
[api_hash] =>
[seo_desc] =>
[jazyk] =>
[jednojazycny] =>
[barva] =>
[indexace] => 1
[obrazek] =>
[ga_force] =>
[cookie_force] =>
[secureredirect] =>
[google_verification] => UOa3DCAUaJJ2C3MuUhI9eR1T9ZNzenZfHPQN4wupOE8
[ga_account] => UA-10822215-3
[ga_domain] =>
[ga4_account] => G-VKDBFLKL51
[gtm_id] =>
[gt_code] =>
[kontrola_pred] =>
[omezeni] =>
[pozadi1] => 0009~~cy6qLC5JzCmOd0zJT0oNLslPzo43NLI0sTA1NLTQLU9NAgA.jpg
[pozadi2] => 0004~~S87PyS_STS6qLC5JzCk2BAA.jpg
[pozadi3] => 0006~~y81PSc2p1C0uKSrNLikt0i1PTQIA.jpg
[pozadi4] => 0008~~c0zJT0oNLslPzo43NTC2NDM1MjbRLU9NAgA.jpg
[pozadi5] =>
[robots] =>
[htmlheaders] =>
[newurl_domain] => 'uach.vscht.cz'
[newurl_jazyk] => 'cs'
[newurl_akce] => '[cs]'
[newurl_iduzel] =>
[newurl_path] => 8548/25669/25670
[newurl_path_link] => Odkaz na newurlCMS
[iduzel] => 25670
[platne_od] => 31.10.2023 17:08:00
[zmeneno_cas] => 31.10.2023 17:08:01.775111
[zmeneno_uzivatel_jmeno] => Jan Kříž
[canonical_url] =>
[idvazba] => 32577
[cms_time] => 1713577712
[skupina_www] => Array
(
)
[slovnik] => stdClass Object
(
[logo_href] => /
[logo] =>  [logo_mobile_href] => /
[logo_mobile] =>
[logo_mobile_href] => /
[logo_mobile] =>  [google_search] => 001523547858480163194:u-cbn29rzve
[social_fb_odkaz] =>
[social_tw_odkaz] =>
[intranet_odkaz] => http://intranet.vscht.cz/
[intranet_text] => Intranet
[mobile_over_nadpis_menu] => Menu
[mobile_over_nadpis_search] => Hledání
[mobile_over_nadpis_jazyky] => Jazyky
[mobile_over_nadpis_login] => Přihlášení
[menu_home] => Domovská stránka
[aktualizovano] => Aktualizováno
[autor] => Autor
[paticka_mapa_odkaz] => Zobrazit mapu
[paticka_budova_a_nadpis] => BUDOVA A
[paticka_budova_a_popis] => Rektorát, oddělení komunikace, pedagogické oddělení, děkanát FCHT, centrum informačních služeb
[paticka_budova_b_nadpis] => BUDOVA B
[paticka_budova_b_popis] => Věda a výzkum, děkanát FTOP, děkanát FPBT, děkanát FCHI, výpočetní centrum, zahraniční oddělení, kvestor
[paticka_budova_c_nadpis] => BUDOVA C
[paticka_budova_c_popis] => Dětský koutek Zkumavka, praktický lékař, katedra ekonomiky a managementu, ústav matematiky
[paticka_budova_1_nadpis] => NÁRODNÍ TECHNICKÁ KNIHOVNA
[paticka_budova_2_nadpis] => STUDENTSKÁ KAVÁRNA CARBON
[paticka_budova_2_popis] =>
[paticka_adresa] => VŠCHT Praha
[google_search] => 001523547858480163194:u-cbn29rzve
[social_fb_odkaz] =>
[social_tw_odkaz] =>
[intranet_odkaz] => http://intranet.vscht.cz/
[intranet_text] => Intranet
[mobile_over_nadpis_menu] => Menu
[mobile_over_nadpis_search] => Hledání
[mobile_over_nadpis_jazyky] => Jazyky
[mobile_over_nadpis_login] => Přihlášení
[menu_home] => Domovská stránka
[aktualizovano] => Aktualizováno
[autor] => Autor
[paticka_mapa_odkaz] => Zobrazit mapu
[paticka_budova_a_nadpis] => BUDOVA A
[paticka_budova_a_popis] => Rektorát, oddělení komunikace, pedagogické oddělení, děkanát FCHT, centrum informačních služeb
[paticka_budova_b_nadpis] => BUDOVA B
[paticka_budova_b_popis] => Věda a výzkum, děkanát FTOP, děkanát FPBT, děkanát FCHI, výpočetní centrum, zahraniční oddělení, kvestor
[paticka_budova_c_nadpis] => BUDOVA C
[paticka_budova_c_popis] => Dětský koutek Zkumavka, praktický lékař, katedra ekonomiky a managementu, ústav matematiky
[paticka_budova_1_nadpis] => NÁRODNÍ TECHNICKÁ KNIHOVNA
[paticka_budova_2_nadpis] => STUDENTSKÁ KAVÁRNA CARBON
[paticka_budova_2_popis] =>
[paticka_adresa] => VŠCHT Praha
Technická 5
166 28 Praha 6 – Dejvice
IČO: 60461373
DIČ: CZ60461373
Datová schránka: sp4j9ch
Copyright VŠCHT Praha 2014
Za informace odpovídá Oddělení komunikace, technický správce Výpočetní centrum
[paticka_odkaz_mail] => mailto:Ondrej.Muller@vscht.cz
[zobraz_desktop_verzi] => zobrazit plnou verzi
[social_fb_title] =>
[social_yt_title] =>
[drobecky] => Nacházíte se: VŠCHT Praha – FCHT – Ústav anorganické chemie
[stahnout] => Stáhnout
[zobraz_mobilni_verzi] => zobrazit mobilní verzi
[social_yt_odkaz] =>
[paticka_budova_1_popis] =>
[social_tw_title] =>
[more_info] => více informací
[den_kratky_2] => út
[novinky_kategorie_1] => Akce VŠCHT Praha
[novinky_kategorie_2] => Důležité termíny
[novinky_kategorie_3] => Studentské akce
[novinky_kategorie_4] => Zábava
[novinky_kategorie_5] => Věda
[novinky_archiv_url] => /novinky
[novinky_servis_archiv_rok] => Archiv z roku
[novinky_servis_nadpis] => Nastavení novinek
[novinky_dalsi] => zobrazit další novinky
[novinky_archiv] => Archiv novinek
[archiv_novinek] => Archiv novinek
[den_kratky_6] => so
[den_kratky_3] => st
[den_kratky_0] => ne
[nepodporovany_prohlizec] => Ve Vašem prohlížeči se nemusí vše zobrazit správně. Pro lepší zážitek použijte jiný.
[den_kratky_1] => po
[preloader] => Prosím čekejte...
[zobrazit_kalendar] => zobrazit kalendář
[zobrazit_vice_kalendar] => více zde →
[hledani_nadpis] => hledání
[hledani_nenalezeno] => Nenalezeno...
[hledani_vyhledat_google] => vyhledat pomocí Google
[den_kratky_4] =>
[den_kratky_5] =>
[social_in_odkaz] =>
[social_li_odkaz] =>
)
[poduzel] => stdClass Object
(
[25672] => stdClass Object
(
[obsah] =>
[poduzel] => stdClass Object
(
[25678] => stdClass Object
(
[obsah] =>
[iduzel] => 25678
[canonical_url] => //uach.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[25677] => stdClass Object
(
[obsah] =>
[iduzel] => 25677
[canonical_url] => //uach.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[25676] => stdClass Object
(
[obsah] =>
[iduzel] => 25676
[canonical_url] => //uach.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
)
[iduzel] => 25672
[canonical_url] =>
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[25673] => stdClass Object
(
[obsah] =>
[poduzel] => stdClass Object
(
[25679] => stdClass Object
(
[nazev] => Ústav anorganické chemie
[seo_title] => Ústav anorganické chemie VŠCHT Praha
[seo_desc] =>
[autor] => ÚACH
[autor_email] =>
[obsah] => Novinky ve formě boxů.
[iduzel] => 25679
[canonical_url] =>
[skupina_www] => Array
(
)
[url] => /home
[sablona] => stdClass Object
(
[class] => stranka_novinky
[html] =>
[css] =>
[js] =>
[autonomni] => 1
)
)
[73595] => stdClass Object
(
[nazev] => SoferGroup
[seo_title] => SoferGroup
[seo_desc] =>
[autor] =>
[autor_email] =>
[obsah] =>
[urlnadstranka] =>
[ogobrazek] =>
[pozadi] =>
[iduzel] => 73595
[canonical_url] =>
[skupina_www] => Array
(
)
[url] => /sofergroup
[sablona] => stdClass Object
(
[class] => boxy
[html] =>
[css] =>
[js] => $(function() {
setInterval(function () { $('*[data-countdown]').each(function() { CountDownIt('#'+$(this).attr("id")); }); },1000);
setInterval(function () { $('.homebox_slider:not(.stop)').each(function () { slide($(this),true); }); },5000);
});
function CountDownIt(selector) {
var el=$(selector);foo = new Date;
var unixtime = el.attr('data-countdown')*1-parseInt(foo.getTime() / 1000); if(unixtime<0) unixtime=0;
var dnu = 1*parseInt(unixtime / (3600*24)); unixtime=unixtime-(dnu*(3600*24));
var hodin = 1*parseInt(unixtime / (3600)); unixtime=unixtime-(hodin*(3600));
var minut = 1*parseInt(unixtime / (60)); unixtime=unixtime-(minut*(60));
if(unixtime<10) {unixtime='0'+unixtime;}
if(dnu<10) {unixtime='0'+dnu;}
if(hodin<10) {unixtime='0'+hodin;}
if(minut<10) {unixtime='0'+minut;}
el.html(dnu+':'+hodin+':'+minut+':'+unixtime);
}
function slide(el,vlevo) {
if(el.length<1) return false; var leva=el.find('.content').position().left; var sirka=el.width(); var pocet=el.find('.content .homebox').length-1;
var cislo=leva/sirka*-1; if(vlevo) { if(cislo+1>pocet) cislo=0; else cislo++; } else { if(cislo==0) cislo=pocet-1; else cislo--; }
el.find('.content').animate({'left':-1*cislo*sirka});
el.find('.slider_puntiky a').removeClass('selected');
el.find('.slider_puntiky a.puntik'+cislo).addClass('selected');
return false;
}
function slideTo(el,cislo) {
if(el.length<1) return false; var sirka=el.width(); var pocet=el.find('.content .homebox').length-1; if(cislo<0 || cislo>pocet) return false;
el.find('.content').animate({'left':-1*cislo*sirka});
el.find('.slider_puntiky a').removeClass('selected');
el.find('.slider_puntiky a.puntik'+cislo).addClass('selected');
return false;
}
[autonomni] => 1
)
)
[25765] => stdClass Object
(
[nazev] => O ústavu
[seo_title] => O ústavu
[seo_desc] =>
[autor] =>
[autor_email] =>
[perex] =>
[ikona] =>
[obrazek] => S60oSS3KTC0yNAIA.jpg
[obsah] => Dnešní Ústav anorganické chemie (před rokem 1989 Katedra anorganické chemie) vznikl po osamostatnění VŠCHT v roce 1952 a zajišťoval výuku předmětu Anorganická chemie a Laboratorní cvičení z anorganické chemie.
Ve vědecko-výzkumné činnosti se pod vedením prof. F. Petrů systematicky zabýval chemií skandia a vzácných zemin. Odborným růstem vědeckých a pedagogických pracovníků ústavu došlo k postupně tematické diferenciaci a vytvoření několika dalších pracovních týmů, zejména po roce 1975, kdy se Ústav podílel na vypracování koncepce nového studijného oboru Chemická technologie kovových a speciálních anorganických materiálů a stal se školícím pracovištěm PGS v oboru anorganické chemie.
Vědeckovýzkumná činnost byla zaměřena na výzkum struktury pevných látek a studium vztahu mezi strukturou a fyzikálně-chemickými vlastnostmi, na výzkum syntézy a aplikací některých anorganických materiálů, zejména tvrdých a abrazivních látek, supravodičů, nekrystalických materiálů, pigmentů a luminoforů, jakož i na výzkum struktury a vlastností koordinačních sloučenin.
V současné době představují významný výzkumný potenciál pracovní skupiny zaměřené na biokoordinační chemii, vysokoteplotní supravodiče, syntetické tvrdé látky, výzkum interakce látek v nízkoteplotním plazmatu a optoelektronické materiály. Především v těchto oborech, které mají podporu z domácích i zahraničních grantových agentur, jsou vypsána témata pro PGS.
[iduzel] => 25765
[canonical_url] => //uach.vscht.cz/o-ustavu
[skupina_www] => Array
(
)
[url] => /o-ustavu
[sablona] => stdClass Object
(
[class] => stranka_obrazek
[html] =>
[css] =>
[js] =>
[autonomni] => 1
)
)
[25766] => stdClass Object
(
[nazev] => Výuka
[seo_title] => Výuka
[seo_desc] =>
[autor] => -
[autor_email] =>
[obsah] =>
| E-learning |
| Portál anorganické chemie |
| Interaktivní výukové aplikace |
Studium v zahraničí
Nabídka zahraničních diplmových a dizertačních prací
[iduzel] => 25766 [canonical_url] => [skupina_www] => Array ( ) [url] => /vyuka [sablona] => stdClass Object ( [class] => stranka_galerie [html] => [css] => [js] => [autonomni] => 1 ) ) [25767] => stdClass Object ( [nazev] => Výzkum [seo_title] => Výzkum [seo_desc] => [autor] => [autor_email] => [obsah] => [iduzel] => 25767 [canonical_url] => //uach.vscht.cz/vyzkum [skupina_www] => Array ( ) [url] => /vyzkum [sablona] => stdClass Object ( [class] => boxy [html] => [css] => [js] => $(function() { setInterval(function () { $('*[data-countdown]').each(function() { CountDownIt('#'+$(this).attr("id")); }); },1000); setInterval(function () { $('.homebox_slider:not(.stop)').each(function () { slide($(this),true); }); },5000); }); function CountDownIt(selector) { var el=$(selector);foo = new Date; var unixtime = el.attr('data-countdown')*1-parseInt(foo.getTime() / 1000); if(unixtime<0) unixtime=0; var dnu = 1*parseInt(unixtime / (3600*24)); unixtime=unixtime-(dnu*(3600*24)); var hodin = 1*parseInt(unixtime / (3600)); unixtime=unixtime-(hodin*(3600)); var minut = 1*parseInt(unixtime / (60)); unixtime=unixtime-(minut*(60)); if(unixtime<10) {unixtime='0'+unixtime;} if(dnu<10) {unixtime='0'+dnu;} if(hodin<10) {unixtime='0'+hodin;} if(minut<10) {unixtime='0'+minut;} el.html(dnu+':'+hodin+':'+minut+':'+unixtime); } function slide(el,vlevo) { if(el.length<1) return false; var leva=el.find('.content').position().left; var sirka=el.width(); var pocet=el.find('.content .homebox').length-1; var cislo=leva/sirka*-1; if(vlevo) { if(cislo+1>pocet) cislo=0; else cislo++; } else { if(cislo==0) cislo=pocet-1; else cislo--; } el.find('.content').animate({'left':-1*cislo*sirka}); el.find('.slider_puntiky a').removeClass('selected'); el.find('.slider_puntiky a.puntik'+cislo).addClass('selected'); return false; } function slideTo(el,cislo) { if(el.length<1) return false; var sirka=el.width(); var pocet=el.find('.content .homebox').length-1; if(cislo<0 || cislo>pocet) return false; el.find('.content').animate({'left':-1*cislo*sirka}); el.find('.slider_puntiky a').removeClass('selected'); el.find('.slider_puntiky a.puntik'+cislo).addClass('selected'); return false; } [autonomni] => 1 ) ) [25768] => stdClass Object ( [nazev] => Vybavení [seo_title] => Vybavení [seo_desc] => [autor] => [autor_email] => [obsah] => [iduzel] => 25768 [canonical_url] => //uach.vscht.cz/vybaveni [skupina_www] => Array ( ) [url] => /vybaveni [sablona] => stdClass Object ( [class] => boxy [html] => [css] => [js] => $(function() { setInterval(function () { $('*[data-countdown]').each(function() { CountDownIt('#'+$(this).attr("id")); }); },1000); setInterval(function () { $('.homebox_slider:not(.stop)').each(function () { slide($(this),true); }); },5000); }); function CountDownIt(selector) { var el=$(selector);foo = new Date; var unixtime = el.attr('data-countdown')*1-parseInt(foo.getTime() / 1000); if(unixtime<0) unixtime=0; var dnu = 1*parseInt(unixtime / (3600*24)); unixtime=unixtime-(dnu*(3600*24)); var hodin = 1*parseInt(unixtime / (3600)); unixtime=unixtime-(hodin*(3600)); var minut = 1*parseInt(unixtime / (60)); unixtime=unixtime-(minut*(60)); if(unixtime<10) {unixtime='0'+unixtime;} if(dnu<10) {unixtime='0'+dnu;} if(hodin<10) {unixtime='0'+hodin;} if(minut<10) {unixtime='0'+minut;} el.html(dnu+':'+hodin+':'+minut+':'+unixtime); } function slide(el,vlevo) { if(el.length<1) return false; var leva=el.find('.content').position().left; var sirka=el.width(); var pocet=el.find('.content .homebox').length-1; var cislo=leva/sirka*-1; if(vlevo) { if(cislo+1>pocet) cislo=0; else cislo++; } else { if(cislo==0) cislo=pocet-1; else cislo--; } el.find('.content').animate({'left':-1*cislo*sirka}); el.find('.slider_puntiky a').removeClass('selected'); el.find('.slider_puntiky a.puntik'+cislo).addClass('selected'); return false; } function slideTo(el,cislo) { if(el.length<1) return false; var sirka=el.width(); var pocet=el.find('.content .homebox').length-1; if(cislo<0 || cislo>pocet) return false; el.find('.content').animate({'left':-1*cislo*sirka}); el.find('.slider_puntiky a').removeClass('selected'); el.find('.slider_puntiky a.puntik'+cislo).addClass('selected'); return false; } [autonomni] => 1 ) ) [10947] => stdClass Object ( [nazev] => Přístup odepřen (chyba 403) [seo_title] => Přístup odepřen [seo_desc] => Chyba 403 [autor] => [autor_email] => [perex] => [ikona] => zamek [obrazek] => [ogobrazek] => [pozadi] => [obsah] =>Nemáte přístup k obsahu stránky.
Zkontrolujte, zda jste v síti VŠCHT Praha, nebo se přihlaste (v pravém horním rohu stránek).
[urlnadstranka] => [iduzel] => 10947 [canonical_url] => [skupina_www] => Array ( ) [url] => /[error403] [sablona] => stdClass Object ( [class] => stranka_ikona [html] => [css] => [js] => [autonomni] => 1 ) ) [1485] => stdClass Object ( [nazev] => Stránka nenalezena [seo_title] => Stránka nenalezena (chyba 404) [seo_desc] => Chyba 404 [autor] => [autor_email] => [obsah] =>Chyba 404
Požadovaná stránka se na webu (již) nenachází. Kontaktuje prosím webmastera a upozorněte jej na chybu.
Pokud jste změnili jazyk stránek, je možné, že požadovaná stránka v překladu neexistuje. Pro pokračování prosím klikněte na home.
Děkujeme!
[urlnadstranka] => [ogobrazek] => [pozadi] => [iduzel] => 1485 [canonical_url] => [skupina_www] => Array ( ) [url] => /[error404] [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) ) ) [iduzel] => 25673 [canonical_url] => [skupina_www] => Array ( ) [url] => [sablona] => stdClass Object ( [class] => [html] => [css] => [js] => [autonomni] => ) ) [519] => stdClass Object ( [nadpis] => [data] => [poduzel] => stdClass Object ( [61411] => stdClass Object ( [nadpis] => [apiurl] => https://studuj-api.cis.vscht.cz/cms/?weburl=/sis [urlwildcard] => cis-path [iduzel] => 61411 [canonical_url] => [skupina_www] => Array ( ) [url] => /sis [sablona] => stdClass Object ( [class] => api_html [html] => [css] => [js] => [autonomni] => 1 ) ) ) [iduzel] => 519 [canonical_url] => [skupina_www] => Array ( ) [url] => [sablona] => stdClass Object ( [class] => [html] => [css] => [js] => [autonomni] => ) ) ) [sablona] => stdClass Object ( [class] => web [html] => [css] => [js] => [autonomni] => 1 ) [api_suffix] => )DATA
stdClass Object
(
[nazev] => Koordinační chemie
[seo_title] => Koordinační chemie
[seo_desc] =>
[autor] =>
[autor_email] =>
[obsah] => Skupina koordinační chemie se zaměřuje na syntézu a charakterizaci koordinačních (např. deriváty vit. B) a organokovových (např. karbenových) sloučenin. Nově také na studium flavinových derivátů, excitovaných stavů molekul a zahrnutí anharmonických efektů ve vibračních spektrech.
Tématikou komplexů biogenních přechodných kovů s ligandy typu Schiffových bazí odvozených od vitaminu B a vybraných aminokyselin (např. alanin, phenylalanin, serin apod.) se zabýváme pro jejich významné cytotoxické vlastnosti. Tyto komplexy také slouží jako modelová biologicky relevantní reakční centra (např. při deaminaci aminokyselin).
Vliv substituce, centrálního atomu kovu a koordinační geometrie na redoxní vlastnosti karbenových komplexů Cr, Fe a W studujeme pomocí elektrochemických a spektroelektrochemických metod. Tyto látky jsou syntetizovány ve spolupráci se skupinou prof. D. Dvořáka z ÚOCH VŠCHT. Experimentální závěry jsou podpořeny DFT výpočty, na kterých spolupracujeme s ÚFCH-JH AV ČR.
Náš výzkum je podporován řadou grantů - RVO MŠMT, GA ČR a IGA VŠCHT, a také granty v rámci spolupráce s Ústavem organické chemie VŠCHT a s Ústavem fyzikální chemie J. Heyrovského AV ČR.
Výzkumný tým
Vedoucí skupiny
Odborní asistenti
Ing. Jan Holub, Ph.D.
Ing. Hana Kotoučová, Ph.D.
Ing. Martin Pižl, Ph.D.
PGS
Ing. Alice Kulagová
Laboranti
Alena Králová
[submenuno] =>
[urlnadstranka] =>
[ogobrazek] =>
[pozadi] =>
[newurl_domain] => 'uach.vscht.cz'
[newurl_jazyk] => 'cs'
[newurl_akce] => '/vyzkum/koordinacni-chemie'
[newurl_iduzel] => 28744
[newurl_path] => 8548/25669/25670/25673/25767/28744
[newurl_path_link] => Odkaz na newurlCMS
[iduzel] => 28744
[platne_od] => 14.09.2022 09:42:00
[zmeneno_cas] => 14.09.2022 09:50:43.700594
[zmeneno_uzivatel_jmeno] => Martin Pižl
[canonical_url] =>
[idvazba] => 36619
[cms_time] => 1713577184
[skupina_www] => Array
(
)
[slovnik] => Array
(
)
[poduzel] => stdClass Object
(
[29117] => stdClass Object
(
[nazev] => Lidé
[seo_title] => Lidé
[seo_desc] =>
[autor] =>
[autor_email] =>
[obsah] => Vedoucí skupiny
Odborní asistenti
Ing. Jan Holub, Ph.D.
Ing. Hana Kotoučová, Ph.D.
Ing. Martin Pižl, Ph.D.
PGS studenti
Ing. Alice Kulagová
Laboranti
Alena Králová
[urlnadstranka] =>
[ogobrazek] =>
[pozadi] =>
[poduzel] => stdClass Object
(
[37212] => stdClass Object
(
[nazev] => Miroslava Guricová
[seo_title] => Miroslava Guricová
[seo_desc] =>
[autor] => Martin Pižl
[autor_email] =>
[obsah] => Kontaktní údaje:
VŠCHT
Místnost A213
e-mail
Výuka:
Chemické výpočty
Úvod do laboratorních výpočtů
Laboratoře anorganické chemie I
Výzkum:
- syntéza koordinačních sloučenin (ligandy = deriváty vitamínů B)
- elektrochemie a spektroelektrochemie karbénových komplexů
Téma dizertační práce:
Koordinační sloučeniny přechodných kovů s redoxně aktivními ligandy odvozenými od vitaminu B6
Diplomová práce:
Komplexy přechodných kovů s redoxně aktivními ligandy odvozenými od vitaminu B6
Vybrané publikace
- Guricová, M.;Pižl, M.; Smékal, Z.; Nádherný, L.; Čejka, J.; Eigner, V.; Hoskovcová, I., Template synthesis and structure of Co(II), Ni(II), and Cu(II) complexes with pyridoxilydenetaurinate Schiff base ligand. Inorganica Chimica Acta 2018, 477, 248-25
- Pižl, M.; Jankovský, O.; Guricová, M.; Hoskovcová, I.; Sedmidubský, D.; Bartůněk, V. Mixed Yttrium–Ytterbium–Erbium Schiff Base Complex as a Model Precursor for Mixed Nanosized Rare Earths Oxides. Journal of Cluster Science 2018, 29 (4), 549-553
Vybrané konference
- Guricová, M; Guldanová, R.; Tobrman, T.; Dvořák, D.; Hoskovcová, I.; 69th Annual ISE Meeting, Bologna (I); 2.-7.9. 2018
- Pižl, M.; Guricová, M.; Hoskovcová, I.; Záliš, S.; Modeling Interactions in Biomolecules VIII; Plzeň (CZ); 3.-8.9.2017
- Guricová, M.; Hoskovcová, I.; Guldanová, R.; Tobrman, T.; Dvořák, D.; Ludvík, J.; 50th Heyrovsky disscusion - Molecular Electrochemistry in Organic and Organometallic Research; Třešť (CZ); 18.-22.6. 2017
- Guricová, M.; Smékal, Z.; Hoskovcová, I.; 68. sjezd chemiků; Praha (CZ); 4.-7.9. 2016
Kontakt
místnost A203
Curiculum vitae
email
Více informací najdete na anglické verzi.
Vypsané práce
Možnost témat naleznete níže nebo případně neváhejte kontaktovat osobně/mailem.
Bakalářské práce:
- Supramolekulární gridy jako funkční stavební bloky pro přípravu „chytrých“ polymerů
- Příprava a studium organorutheniových komplexů pro elektrokatalyzovaný rozklad vody a ammoniaku se zaměřením na udržitelnou výrobu „zeleného“ vodíku
- Studium různých koordinačních motivů pro světlem kontrolovanou výměnu ligandů s ohledem na možné budoucí využití při doručování bioaktivních látek a léčiv
- Syntéza a studium rovnováhy koordinačních sloučenin jako kombinatoriálních motivů zprostředkovávajích interakci mezi různými proteiny
Magisterské práce:
- Self-assembling surface platforms for catalysis and sensing based on supramolecular grids
Zaměření
Naše skupina se věnuje koordinační chemii a využití organokovových komplexů pro praktické využití jak v materiálové chemie tak v elektrokatalýze pro zelené transformace základních látek (H2O, CO2). Naše chemie leží na hranici mezi organickou a anorganickou chemii což znamená, že si studenti vyzkouší jak organickou syntézu tak i praktické měření fyzikálně-chemických vlastností spojených s kovy. Díky přístrojovému vybavení naší laboratoře využíváme spektrofotometrická měření, elektrochemii a jejich rozličné kombinace. Pokud Vás lákají komplexní problémy a úkoly a chcete se všestraně chemicky rozvíjet jak v syntéze tak v práci s přístroji – Přijďte k nám, rádi Vás uvítáme!
Výzkum
[urlnadstranka] => [iduzel] => 63019 [canonical_url] => [skupina_www] => Array ( ) [url] => /vyzkum/koordinacni-chemie/lide/holub [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) ) [44628] => stdClass Object ( [nazev] => Viera Murašková [seo_title] => Viera Murašková [seo_desc] => [autor] => [autor_email] => [obsah] =>Kontaktní údaje
VŠCHT
Místnost A213, BS45
e-mail
Výuka
Obecná a anorganická chemie I - cvičení
Obecná a anorganická chemie II - cvičení
Laboratoř anorganické chemie I
Laboratoř anorganické chemie II
Laboratoře oboru chemie a technologie materiálů
Výzkum
- Syntéza a charakterizace komplexů Fe(III) s ligandy typu Schiffových bází odvozených od pyridoxalu
Vybrané publikace
- Murašková, V.; Dušek, M.; Buryi, M.; Laguta, V.; Huber, Š.; Sedmidubský, D. Synthesis, characterization and X-ray crystal structure of an iron(III) complex of a tripodal pyridoxal Schiff base ligand: effects of positional disorder on its magnetic properties, Transit. Met. Chem. 2018, 43, 605-619
- Murašková, V.; Szabó, N.; Pižl, M.; Hoskovcová, I.; Dušek, M.; Huber, Š.; Sedmidubský, D. Self assembly of dialkoxo bridged dinuclear Fe(III) complex of pyridoxal Schiff base with CC bond formation – Structure, spectral and magnetic properties, Inorganica Chim. Acta. 2017, 461, 111–119
Vybrané konference
- Murašková, V.; Dušek, M.; Sedmidubský, D. 8th European Chemistry Congress, Paris (F), 21. – 23.6. 2018
- Murašková, V.; Pižl, M.; Hoskovcová, I. 1. Pokroky anorganické chemie, Třešť (CZ), 22. – 26. 6. 2014
- Murašková, V.; Szabó, N.; Hoskovcová, I. XXIV. International Conference on Coordination and Bioinorganic Chemistry, Smolenice (SVK), 2. – 7.6. 2013

Kontaktní údaje
VŠCHT
Místnost A285, A213
e-mail
Výuka
Obecná a anorganická chemie I - cvičení
Obecná a anorganická chemie II - cvičení
Základy farmakologie
Laboratoř z anorganické chemie I
Výzkum
- Syntéza Fisherových karbenů - fotoreaktivita s iminy
- Syntéza atropoizomerních flavinů - studium redox vlastností a izomeračních (rotačních) energetických bariér
- Syntéza organických ligandů pro vybrané typy komplexů
Vybrané publikace
- Kotoučová, H.; Strnadová, I.; Kovandová, M.; Biomimetic aerobic oxidative hydroxylation of arylboronic acids to phenols catalysed by a flavin derivative. Org. Biomol. Chem., 2014, 13 (12), 2137-2142
- Adamec, M.; Beneš, P.; Kotoučová, H.; Kvalitativní předpověď relativní síly oxokyselin z jejich souhrnných vzorců. Biologie-Chemie-Zeměpis, 2011, 20 (2), 78-83. ISSN 1210-3349.
- Kotoučová, H.; Solnčika, O.; Cibulka, R.; Syntéza a studium vlastností orthosubstituovaných 3-fenylisoalloxazinů. In: Chemické listy 6/2010: 62. sjezd českých a slovenských asociací chemických společností. 1 vyd. Praha: Česká společnost chemická, 2010, s. 433-433
- Kotoučová, H.; Košata, B.; Cibulka, R.; Interaktivní elektronické testy z organické chemie. In: Chemické listy 6/2010: 62. sjezd českých a slovenských asociací chemických společností. 1 vyd. Praha: Česká společnost chemická, 2010, s. 552-552
- Kotoučová, H.; Cibulka, R.; N-arylace flavinů v poloze 3- za podmínek Chanova-Lamova Couplingu. Chemické listy, 2008, 102 (12),
 Kontaktní údaje
Kontaktní údaje
VŠCHT
Místnost A285, A213
e-mail
Výuka
Obecná anorganická chemie I - přednáška, cvičení
Obecná anorganická chemie II - cvičení
Laboratoř anorganické chemie I
Laboratoř oboru
Koordinační chemie
Bioanorganická chemie
Výzkum
- elektrochemie karbenových sloučenin
Vybrané publikace
- Guricová, M.; Pižl, M.; Smékal, Z.; Nádherný, L.; Čejka, J.; Eigner, V.; Hoskovcová, I., Template synthesis and structure of Co(II), Ni(II), and Cu(II) complexes with pyridoxilydenetaurinate Schiff base ligand. Inorganica Chimica Acta 2018, 477, 248-25
- Váňová H.; Tobrman T.; Hoskovcová I.; Dvořák D. Modular Synthesis of Fischer Biscarbene Complexes of Chromium. Organometallics 2016, 35 (17), 2999–3006
- Metelková R.; Hoskovcová I.; Polášek M.; Urban J.; David T.; Ludvík J. Stereoisomeric products of electrochemical reduction of heterocyclic Fischer aminocarbene Cr(0) complexes. Development of the electrochemistry-mass spectrometry tandem approach using biphasic (acetonitrile-hexane) preparative electrolysis. Electrochimica Acta 2015, 162, 17–23
- Kvapilová H.; Hoskovcová I.; Ludvík J.; Záliš S. Theoretical Predictions of Redox Potentials of Fischer-Type Chromium Aminocarbene Complexes. Organometallics 2014, 33 (18), 4964–4972
Kapitola ve sborníku
- Ludvík J.; Hoskovcová I. Electrochemistry of Fischer aminocarbene complexes: effects of structure on redox properties, electron distribution, and reaction mechanisms, in: Advances in Organometallic Chemistry and catalysis: The silver/gold jubilee International Conference on Organometallic Chemistry celebratory Book, ed. A. J. L. Pombeiro, J.Wiley 2014

Kontaktní údaje
VŠCHT
Místnost A213
e-mail
Výuka
Obecná a anorganická chemie I - cvičení
Laboratoř anorganické chemie I
Výzkum
- syntéza koordinačních sloučenin
- studium excitovaných stavů molekul
- elektrochemie a spektroelektrochemie (kombinace CV s UV-Vis či IR)
- vibrační spektroskopie koordinačních sloučenin (FTIR, TRIR, 2DIR, T-2DIR, Raman)
- kvantově-chemický popis fyzikálně-chemických vlastností koordinačních sloučenin (Gaussian, Orca, Molcas apod.)
Publikace
- Taylor, J. O.; Pižl, M.; Kloz, M.; Rebarz, M.; McCusker, C. E.; McCusker, J. K.; Záliš, S.; Hartl, F.; Vlček, A., Optical and Infrared Spectroelectrochemical Studies of CN-Substituted Bipyridyl Complexes of Ruthenium(II).Inorganic Chemistry 2021, 60 (6), 3514-3523
- Sondermann, C.; Pižl, M.; Paretzki, A.; Feil, C.; Ringenberg, M. R.; Záliš, S.; Kaim, W., Analysis of a Diimine-Organonickel Redox Series. Eur J Inorg Chem 2020, 2020 (31), 3010-3015.
- Pižl, M.; Picchiotti, A.; Rebarz, M.; Lenngren, N.; Yingliang, L.; Záliš, S.; Kloz, M.; Vlček, A., Time-Resolved Femtosecond Stimulated Raman Spectra and DFT Anharmonic Vibrational Analysis of an Electronically Excited Rhenium Photosensitizer. The Journal of Physical Chemistry A 2020, 124 (7), 1253-1265.
- Guricová, M.; Tobrman, T.; Pižl, M.; Žižková, S.; Hoskovcová, I.; Dvořák, D., Synthesis, characterisation and electrochemical properties of Cr(0) aminocarbene complexes containing condensed heteroaromatic moiety. J Organomet Chem 2020, 905, 121023.
- Takematsu, K.; Pospíšil, P.; Pižl, M.; Towrie, M.; Heyda, J.; Záliš, S.; Kaiser, J. T.; Winkler, J. R.; Gray, H. B.; Vlček, A., Hole Hopping Across a Protein–Protein Interface. The Journal of Physical Chemistry B 2019, 123 (7), 1578-1591.
- Chen, L.; Lim, K. J. C.; Babra, T. S.; Taylor, J. O.; Pižl, M.; Evans, R.; Chippindale, A. M.; Hartl, F.; Colquhoun, H. M.; Greenland, B. W. A macrocyclic receptor containing two viologen species connected by conjugated terphenyl groups. Organic & Biomolecular Chemistry 2018, 16 (27), 5006-5015
- Pižl, M.; Jankovský, O.; Guricová, M.; Hoskovcová, I.; Sedmidubský, D.; Bartůněk, V. Mixed Yttrium–Ytterbium–Erbium Schiff Base Complex as a Model Precursor for Mixed Nanosized Rare Earths Oxides. Journal of Cluster Science 2018, 29 (4), 549-553
- Guricová, M.; Pižl, M.; Smékal, Z.; Nádherný, L.; Čejka, J.; Eigner, V.; Hoskovcová, I., Template synthesis and structure of Co(II), Ni(II), and Cu(II) complexes with pyridoxilydenetaurinate Schiff base ligand. Inorganica Chimica Acta 2018, 477, 248-256.
- Pižl, M.; Hunter, B. M.; Greetham, G. M.; Towrie, M.; Záliš, S.; Gray, H. B.; Vlček, A., Ultrafast Wiggling and Jiggling: Ir2(1,8-diisocyanomenthane)42+. The Journal of Physical Chemistry A 2017, 121 (48), 9275-9283.
- Murašková, V.; Szabó, N.; Pižl, M.; Hoskovcová, I.; Dušek, M.; Huber, Š.; Sedmidubský, D., Self assembly of dialkoxo bridged dinuclear Fe(III) complex of pyridoxal Schiff base with CC bond formation – Structure, spectral and magnetic properties. Inorganica Chimica Acta 2017, 461, 111-119.
- Pižl, M.; Jankovský, O.; Ulbrich, P.; Szabó, N.; Hoskovcová, I.; Sedmidubský, D.; Bartůněk, V., Facile preparation of nanosized yttrium oxide by the thermal decomposition of amorphous Schiff base yttrium complex precursor. Journal of Organometallic Chemistry 2017, 830, 146-149.
- Langmaier, J.; Pižl, M.; Samec, Z.; Záliš, S.; Extreme Basicity of Biguanide Drugs in Aqueous Solutions: Ion Transfer Voltammetry and DFT Calculations. The Journal of Physical Chemistry A 2016, 120 (37), 7344-7350.
Vybrané konference
- Pižl, M.; Fuse, M.; Tasinato, N.; Barone,B.; Vlček, A.; Záliš, S.; Photoinduced Processes in Embedded Systems; Pisa (I); 24.-27.6. 2018
- Záliš, S.; Pižl, M.; Fuse, M.; Barone,B.; Vlček, A.; 16th International Congress of Quantum Chemistry; Menton (F); 18.-23.6. 2018
- Záliš, S.; Pižl, M.; Vlček, A.; Gray, H.B.; 7th JCS SYMPOSIUM; Prague (CZ); 21.-24.5. 2018
- Záliš, S.; Pižl, M.; Heyda, J.; Vlček Jr., A.; 3rd MOLIM General Meeting; Budapest (H); 19.-21.4. 2018
- Pižl, M.; Fuse, M.; Barone, V.; Antonín Vlček, A.; Záliš, S.; Anharmonicity in Medium-Sized Molecules and Clusters; Budapest (H); 16.-19.4. 2018
- Pižl, M.; Guricová, M.; Hoskovcová, I.; Záliš, S.; Modeling Interactions in Biomolecules VIII; Plzeň (CZ); 3.-8.9.2017
- Pižl, M.; Záliš, S.; Heyda, J.; Vlček Jr., A.; 11th Triennial Congress of the World Association of Theoretical and Computational Chemists; Munich (D); 27.8.-1.9.2017
- Pižl, M.; Vlček Jr., A.; Towrie, M.; Greetham, G.; Gray, H.B.; Záliš, S.; 22nd International Symposium on Photochemistry and Photophysics of Coordination Compounds; Oxford (UK); 9.-14.7.2017
- Taylor, J.; Pižl, M.; Donaldson, P.; Vlček Jr., A.; Hartl, F.; 22nd International Symposium on Photochemistry and Photophysics of Coordination Compounds; Oxford (UK); 9.-14.7.2017
- Pižl, M.; Záliš, S.; Paretzki, A.; Kaim, W; 50th Heyrovsky disscusion - Molecular Electrochemistry in Organic and Organometallic Research; Třešť (CZ); 18.-22.6.2017
- Záliš, S.; Pižl, M.; Heyda, J.; Vlček Jr., A.; 2nd MOLIM General Meeting; Dubrovník (HR); 10.-12.10.2016
Dizertační práce
Diplomová práce
[submenuno] => 1 [urlnadstranka] => [ogobrazek] => [pozadi] => [iduzel] => 29525 [canonical_url] => [skupina_www] => Array ( ) [url] => /vyzkum/koordinacni-chemie/lide/pizl [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) ) ) [iduzel] => 29117 [canonical_url] => [skupina_www] => Array ( ) [url] => /vyzkum/koordinacni-chemie/lide [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) ) [44861] => stdClass Object ( [nazev] => Přístrojové vybavení [seo_title] => Přístrojové vybavení [seo_desc] => [autor] => MP [autor_email] => [obsah] =>Přístrojové vybavení
Naše laboratoř disponuje vybavením pro elektrochemická měření (potenciostaty Autolab), pro optickou charakterizaci a pro měření FTIR. Další způsoby charakterizace je možné provádět v rámci ústavu (Přístrojové vybavení ústavu) nebo spolupráce s ostatními ústavy.
- Autolab PGSTAT101
- Autolab PGSTAT128N
- Varian Cary 50 - UV-Vis spektrometr
- Nicolet iS5 – pro ATR (iD7) a pro transmisi (iD1) - FTIR
- Vláknový spektrometr Avantes pro UV-Vis oblast
Nabízené práce:
V případě zájmu se na nás obraťte — laboratoř A213.
Bakalářské práce:
- Supramolekulární gridy jako funkční stavební bloky pro přípravu „chytrých“ polymerů (kontakt - Holub)
- Syntéza komplexů přechodných kovů s bipyridinem a jeho deriváty (kontakt - Pižl)
- Příprava a studium organorutheniových komplexů pro elektrokatalyzovaný rozklad vody a ammoniaku se zaměřením na udržitelnou výrobu „zeleného“ vodíku (kontakt - Holub)
- Spektroskopická a elektrochemická studie derivátů vitaminu B6 a jejich interakce s přechodnými kovy (kontakt - Pižl)
- Studium různých koordinačních motivů pro světlem kontrolovanou výměnu ligandů s ohledem na možné budoucí využití při doručování bioaktivních látek a léčiv (kontakt - Holub)
- Syntéza a studium rovnováhy koordinačních sloučenin jako kombinatoriálních motivů zprostředkovávajích interakci mezi různými proteiny (kontakt - Holub)
Magisterské práce:
- Self-assembling surface platforms for catalysis and sensing based on supramolecular grids (kontakt - Holub)
- Spektroelektrochemie komplexů přechodných kovů obsahujících redoxně-aktivní ligandy (kontakt - Pižl)
- Sledování interakce biogenních iontů přechodných kovů s ligandy flavinového typu (kontakt - Kotoučová
Doktorské práce:
- Koordinační sloučeniny přechodných kovů s redoxně aktivními ligandy (kontakt - Hoskovcová)
Řešené práce:
- Elektrooxidace vody a amoniaku pomocí molekulárních katalyzátorů a jejich integrace do heterogenních molekulárních anod - Ing. Alice Kulagová (dizertace; školitel: Holub)
- Interkalátory DNA na bázi α-diiminových komplexů chromu - Anna Mašková (3. ročník Bc.; školitel: Pižl)
- Supramolekulární gridy jako multivaletní základ pro přípravu 1D a 2D struktur - Rebeka Morvaiová (3. ročník Bc.; školitel: Holub)
- Využití CO2 a CO k udržitelné přípravě chemických specialit pomocí elektrochemie a katalýzy přechodných kovů - Karolína Česneková (3. ročník Bc.; školitel: Holub)
- Syntéza kobaltnatých komplexů odvozených od pyridoxalu a jeho 5C-substituentů - Pavlína Malečková (3. ročník Bc.; školitel: Pižl)
Electrosynthesis approach to CO2 and CO capture for preparation of value-added chemicals
Electrochemical Reduction:
Beyond CO2/CO: Synthetic electrochemistry
Under the pressure of global warming and increasing level of CO2, much effort is being made to find a green transformation that can reduce its atmospheric levels. Most of the work is devoted to (electro)chemical reduction of CO2 into CO, HCOOH, CH4 etc. Few reports deal with direct CO2 electrochemical transformations such as carboxylation. Even fewer try to couple CO2 reduction in combination with another chemical transformation. The illustrative example is a report on the two-step CO2/CO reduction process combined with carbonylative Sonogashira or Suzuki couplings.[1] This work aims to couple two independent electrochemical processes in one classic organic reaction. More specifically, coupling a CO2/CO reduction with an oxidative electro-generation of aryne to achieve an arene carbonylation reaction for aldehyde, fluorenone or anthraquinone production. (Figure 1)
Methodology
1) Aryne electrogeneration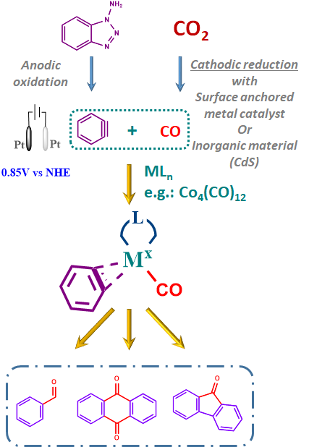
Arynes are reactive and useful intermediates in the synthesis of aromatic compounds; however, due to their reactivity, it is necessary to generate them in situ and react immediately. Many different methodologies are already relying on the stoichiometric use of particular reagents to generate an aryne triple bond. Especially interesting for the scope of this work is a recent study on the electrochemical generation of arynes from 1-aminobenzotriazole for stereoselective arylation of β‐Ketocarbonyls.[2] The protocol takes advantage of a standard electrochemical set-up and commercially available materials providing a solid starting point for further build-up. (Figure 1)
2) CO addition
The direct reaction of CO with arynes is interesting as it provides direct access to anthraquinones and fluorenones. These compounds are widely used moieties in various fields, from medicine to material chemistry or organic electronics.[3,4]
The benzyne carbonylation can be catalysed with cheap first-row transition metals such as cobalt[5], which coincidentally also helps with in-situ aryne stabilisation. Using different metal catalysts (Ni[6], Rh[7]) could then alter the reaction selectivity and allow access to different products. (Figure 1) An important part of this step is optimising the reaction conditions starting from the build-up of the catalyst, solvent, temperature and current. In this regard, an intriguing possibility is also an application of UV light on the solution in the presence or absence of CO. This will trigger the rearrangement of benzyne into ketene and open the door to a possibly different reactivity.[8]
Figure 1: Illustration of the forseen aryne carbonylation process
3) Convert CO2 reduction into aryne carbonylation
Subsequently, the combination of CO2 reduction and aryne generation can be done in two ways: through the sequential line-up and one-pot reaction (Figure 2). The CO2 to CO reduction can be achieved either through electrocatalytic cathodic reduction with transition metal catalysts or using contemporary state-of-the art photoreduction using CdS nanonsheets.9 In the case of the separated reactions, the simpler set-up counts with two (electro)chemical cells connected through a tube bridge, allowing CO to cross from the evolution chamber to the aryne solution (Figure 2a). This set-up offers an experimental simplicity; however, it poses challenges to selectivity as CO2 can enter together with CO. However, issues might appear regarding scalability, time efficiency and the reliance of electrochemical reactions on mass transfer. Finally, the more sophisticated approach uses flow chemistry (Figure 3b). In this set-up, it is possible to maximise the efficiency of both electrochemical processes, avoid selectivity issues and decrease the reaction time while quickly scaling up the output.
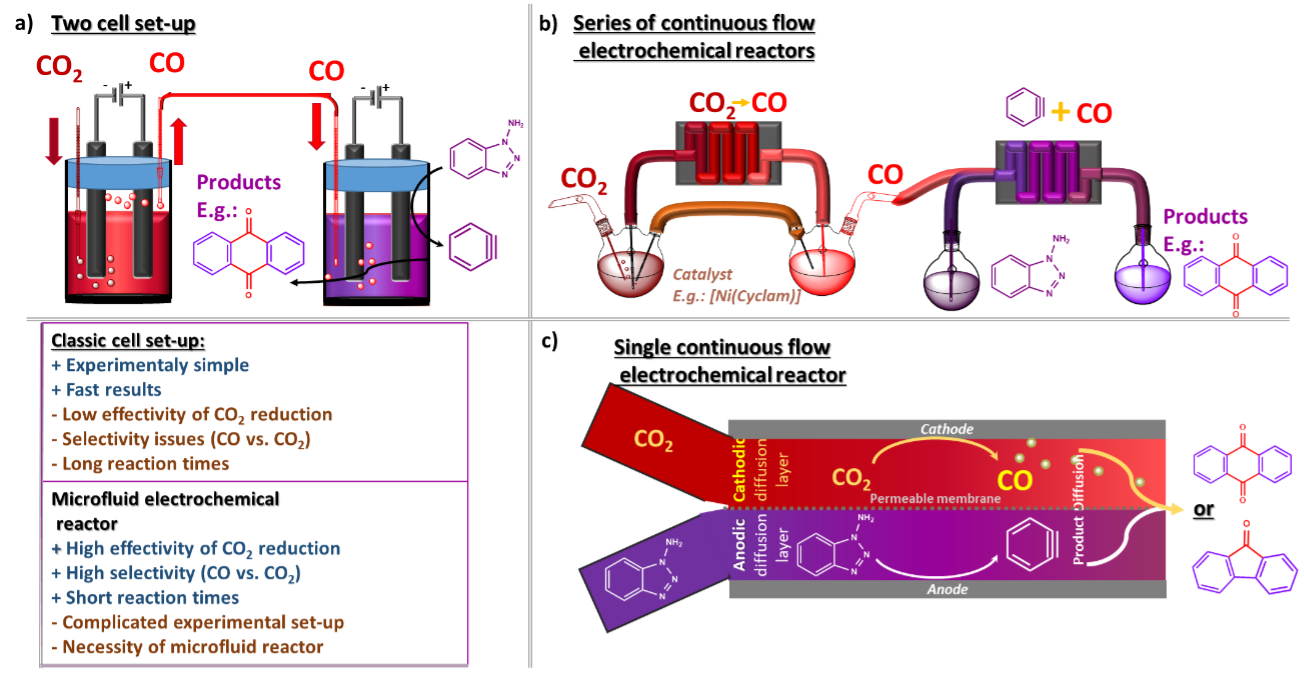
Figure 2: Illustration of different experimental set-ups for effectively coupling two electrochemical reactions into one process. a) Two separate classic electrochemical cells; b) Two microfluidic reactors in series; c) One-pot process in a single microfluidic reactor
The ultimate goal is then to connect both electrochemical processes in one reaction set-up. Again due to the possible competition between CO2 and CO, the prefered approach is to use a chemical flow reactor (Figure 2c). The limited diffusion between the anodic and cathodic layers allows for better effectivity of initial individual transformations, resulting in higher selectivity.[10,11] This is possible simply because, at first, the reactants react in their respective layers. At the same time, the diffusion-conditioned intermolecular reaction happens in distant parts of the reactor where the starting reactants have already transformed into intermediates (e.g. CO2 to CO).
Literature:
[1] M. T. Jensen, M. H. Rønne, A. K. Ravn, R. W. Juhl, D. U. Nielsen, X. M. Hu, S. U. Pedersen, K. Daasbjerg, T. Skrydstrup, Nat. Commun. 2017, 8
[2] L. Li, Y. Li, N. Fu, L. Zhang, S. Luo, Angew. Chemie - Int. Ed. 2020, 59, 14347–14351.
[3] S. Patel, B. Rathod, S. Regu, S. Chak, A. Shard, ChemistrySelect 2020, 5, 10673–10691.
[4] E. M. Malik, C. E. Müller, Med. Res. Rev. 2016, 36, 705–748.
[5] N. Chatani, A. Kamitani, M. Oshita, Y. Fukumoto, S. Murai, J. Am. Chem. Soc. 2001, 123, 12686–12687.
[6] B. L. Edelbach, R. J. Lachicotte, W. D. Jones, Organometallics 1999, 18, 4660–4668.
[7] C. N. Iverson, W. D. Jones, Organometallics 2001, 20, 5745–5750.
[8] J. G. Radziszewski, J. Waluk, P. Kaszynski, J. Spanget-Larsen, J. Phys. Chem. A 2002, 106, 6730–6737.
[9] N. Wang, S. Cheong, D.-E.Yoon, P. Lu, H. Lee, Y. K. Lee, Y.-S. Park, D. C. Lee, J. Am. Chem. Soc. 2022, 144, 16974-16983
[10] T. Noël, Y. Cao, G. Laudadio, Acc. Chem. Res. 2019, 52, 2858–2869.
[11] D. Horii, T. Fuchigami, M. Atobe, J. Am. Chem. Soc. 2007, 129, 11692–11693.
Screening of different coordination motives in phototriggered ligand exchange on metal complexes for targeted drug delivery
Supramolecular stapling of peptides and proteins:
Introduction
Light as a non-invasive and non-destructive force is a highly appreciated and sought way of influencing chemical reactions and physicochemical features of active compounds. It allows precise spatiotemporal focus and is cheap and selective as it influences only artificially tweaked molecules. The current development is hindered by the low penetration depth of the shortest wavelength, where most of the activity is observed.
This field is nowadays dominated by pure organic compounds with photodissociating bonds. However, many photoactive complexes are also known in the literature. These are able, upon irradiation, to release their ligands and rearrange their coordination sphere. Some were already tested as vectors for drug delivery. There, the light triggered the release of the cargo only in the specific and restricted area of light impact. However, phototrigered complexes and their interactions with larger biological structures are still much undeveloped.
In comparison, the coordination compounds offer binding strength, often rivalling covalent bonds. Above that, the presence of metal often brings the interesting prospect of spatiotemporal visualisation through fluorescence or isotopic labelling. This project aims to explore ways of photo-triggered release from coordination compounds for therapeutic purposes, employing this concept in complex and unique modes of action.
The aims and methodology:
In this section, the model modes of action will describe each goal's key steps, and all will be illustrated with specific examples. The projects progressively build in complexity on Top of each other; however, underlying chemical, biological and analytical methods remain the same throughout the proposal.
Light-controlled release from Vanadium complexes
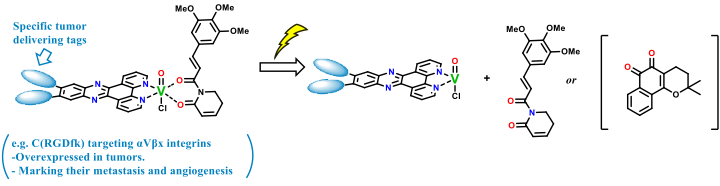
Vanadium complexes were already proven to be able to release their ligands upon light irradiation. The successful work with curcumin as a ligand showed the viability of this approach for a targeted death of tumour cells.1 One can imagine a plethora of other therapeutic compounds to be coordinated and released similarly. In order to learn the methodology and synthetic procedures, this project will start with preparing a library of Vanadium complexes with different motives, such as Piperlongumine (anticancer inhibitor) and Lapachone (ROS generation), which susceptibility towards the light will be tested.
Targeted release
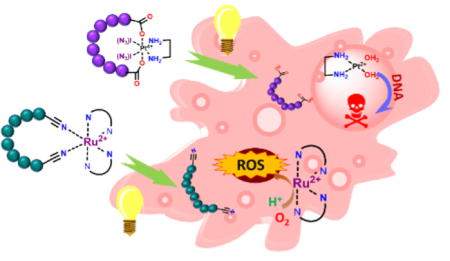 Later, when the basic methodology is mastered, more advanced topics will be studied, including short polypeptides stapled/cyclised with Ruthenium or platinum. Such complexes are relevant in various anticancer therapies. For example, Ruthenium2,3 is known to form reactive oxygen species, while platinum4 drugs are potent intercalators to DNA, both causing cell apoptosis. However, both families are highly unspecific in their cellular uptake, consequently having high off-target impact and toxicity. Connection with cell-specific delivering tags stapled together in a photouncaging complex would lead to a double layer of specificity, ensuring that only the irradiated cells are affected by the therapy. The ligand functionalisation could also be used to shift the active wavelength into a two-photon absorption range and the infrared region with considerably deeper penetration depth.
Later, when the basic methodology is mastered, more advanced topics will be studied, including short polypeptides stapled/cyclised with Ruthenium or platinum. Such complexes are relevant in various anticancer therapies. For example, Ruthenium2,3 is known to form reactive oxygen species, while platinum4 drugs are potent intercalators to DNA, both causing cell apoptosis. However, both families are highly unspecific in their cellular uptake, consequently having high off-target impact and toxicity. Connection with cell-specific delivering tags stapled together in a photouncaging complex would lead to a double layer of specificity, ensuring that only the irradiated cells are affected by the therapy. The ligand functionalisation could also be used to shift the active wavelength into a two-photon absorption range and the infrared region with considerably deeper penetration depth.
Initial in-vitro studies of photo release can be done by standard analytical techniques such as NMR, UV, and HPLC. The complexes will then be fed to cell cultures, and the IC50 of different combinations will be measured in the dark or under irradiation and analysed. The added possibility is to use isotopic labelling or fluorescent ligands to follow the journey through the organism.
Targeted delivery using accumulated metals as photosensitizers
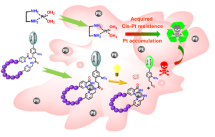 Furthermore, it is a well-known fact that some cancer types and diseased cells accumulate with increasing resistance certain metal cations such as Copper or Platinum. Here we would like to use the specific accumulation of platinum in cis-platinum-resistant cancer cultures. The peptidic delivering/active part will be stapled with a specific organic motive.5,6 Onto this motive, a specific active moiety will be grafted. The metal complexation will then happen directly in the affected cell and serve as a sensitisation method and activator for light-mediated release. At this stage, we will combine supramolecular chemistry with classic organic chemistry to reach cell-specific effects, hopefully obtaining the ability to differentiate between healthy and pathological cancer cells. The work will start with the incorporation of fluorescent dye to study the delivery and release in-vitro and in-vivo. Later, the same experiments will be repeated with model active compounds to achieve the desired therapeutic effect.
Furthermore, it is a well-known fact that some cancer types and diseased cells accumulate with increasing resistance certain metal cations such as Copper or Platinum. Here we would like to use the specific accumulation of platinum in cis-platinum-resistant cancer cultures. The peptidic delivering/active part will be stapled with a specific organic motive.5,6 Onto this motive, a specific active moiety will be grafted. The metal complexation will then happen directly in the affected cell and serve as a sensitisation method and activator for light-mediated release. At this stage, we will combine supramolecular chemistry with classic organic chemistry to reach cell-specific effects, hopefully obtaining the ability to differentiate between healthy and pathological cancer cells. The work will start with the incorporation of fluorescent dye to study the delivery and release in-vitro and in-vivo. Later, the same experiments will be repeated with model active compounds to achieve the desired therapeutic effect.
Literature:
1) Banerjee S, Prasad P, Hussain A, Khan I, Kondaiah P, Chakravarty AR. Remarkable photocytotoxicity of curcumin in HeLa cells in visible light and arresting its degradation on oxovanadium(IV) complex formation. Chem Commun (Camb). 2012 Aug 11;48(62):7702-4. doi: 10.1039/c2cc33576j.
2) Rapp TL, Wang Y, Delessio MA, Gau MR, Dmochowski IJ. Designing Photolabile Ruthenium Polypyridyl Crosslinkers for Hydrogel Formation and Multiplexed, Visible-light Degradation. RSC Adv. 2019;9(9):4942-4947. doi: 10.1039/C8RA09764J.
3) Qu F , Martinez K , Arcidiacono AM , Park S , Zeller M , Schmehl RH , Paul JJ , Kim Y , Papish ET Sterically demanding methoxy and methyl groups in ruthenium complexes lead to enhanced quantum yields for blue light triggered photodissociation. Dalton Trans. 2018 Nov 13;47(44):15685-15693. doi: 10.1039/c8dt03295e.
4) Stefanie Perfahl, Marta M. Natile, Heba S. Mohamad, Christiane A. Helm, Carola Schulzke, Giovanni Natile, and Patrick J. Bednarski Molecular Pharmaceutics 2016 13 (7), 2346-2362 DOI: 10.1021/acs.molpharmaceut.6b00108
5) Ciesienski, K.L., Hyman, L.M., Yang, D.T., Haas, K.L., Dickens, M.G., Holbrook, R.J. and Franz, K.J. (2010), A Photo-Caged Platinum(II) Complex That Increases Cytotoxicity upon Light Activation. Eur. J. Inorg. Chem., 2010: 2224-2228. https://doi.org/10.1002/ejic.201000098
6) Kumbhar AA, Franks AT, Butcher RJ, Franz KJ. Light uncages a copper complex to induce nonapoptotic cell death. Chemical Communications (Cambridge, England). 2013 Mar;49(24):2460-2462. DOI: 10.1039/c3cc38927h
[urlnadstranka] => [ogobrazek] => [pozadi] => [iduzel] => 67580 [canonical_url] => [skupina_www] => Array ( ) [url] => /vyzkum/koordinacni-chemie/prace/drug_delivery [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) ) [67579] => stdClass Object ( [nazev] => [seo_title] => Testing the possibility of organometalic coordination complexes in preparation of bifunctional ligands modifying protein-protein interactions [seo_desc] => [autor] => [autor_email] => [obsah] =>Testing the possibility of organometalic coordination complexes in preparation of bifunctional ligands modifying protein-protein interactions
Dynamic Combinatorial approach to PROTACs: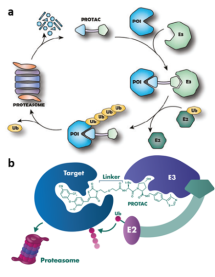
PROTACs and its mode of action
In recent years PROATACs gained much attention due to their ability to target previously "undruggable" substrates.1,2 The concept is used to label a protein of interest (PI) with ubiquitin to trigger its proteasome degradation. (Figure 1a, Top) PROTACs molecules are heterobifunctional compounds/ligands with two active parts connected through an organic linker. In a simplified way, one of the ligand's heads binds to PI intended for degradation. The other head then connects to another protein responsible for degradation. The result is a precise clearance of a particular PI, moderation of all its downstream processes and ultimately, a correction of associated pathological processes.
However, there are also certain limitations to the current PROTAC ligands. Since they must bear two, often big, binding units and an appropriately long organic linker, the size of the molecule can become a problem.5 If too big, it can lead to a lower uptake. Secondly, if the concentration of the PROTAC ligand is higher, the activity decreases due to the competitiveness and saturation of binding sites, resulting in a characteristic bell-shaped curve.6 (Figure 2, Left)
Thirdly, the covalent nature of ligands is a problem for scaffold optimisation and modification. Therefore, any change in either warheads, linker size or target PI requires lengthy synthesis of a whole ligand.
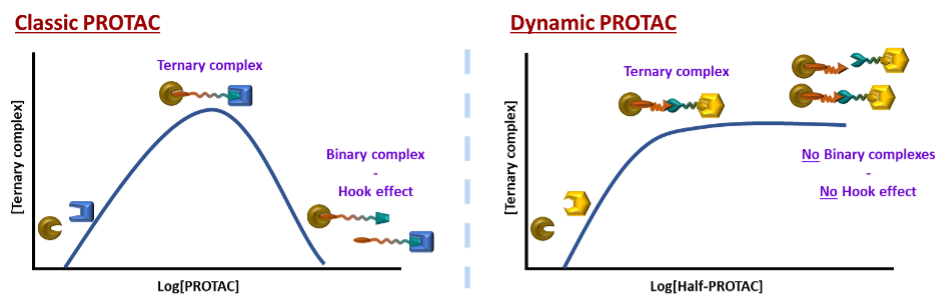
Figure 2: Left: The bell curve of the concentration dependence of Classic Protacs; Right: Foreseen improved concentration curve of dynamic PROTACs with no decrease of effectivity at higher concentrations
Dynamic combinatorial PROTACs
However, introducing a new type of dynamic linking motive could solve all the original issues. This way, the size of the delivered molecule decreases as only half-sized molecules must be delivered into a cell. The dynamic combinatorial way means a statistical formation of productive heterobifunctional specie. Therefore, since the binding events of the two heads are now independent and do not interfere with each other, there will be no hook effect, no bell-shaped concentration curve and consequently, less stringent concentration limitations. (Figures 2 Left and 3).
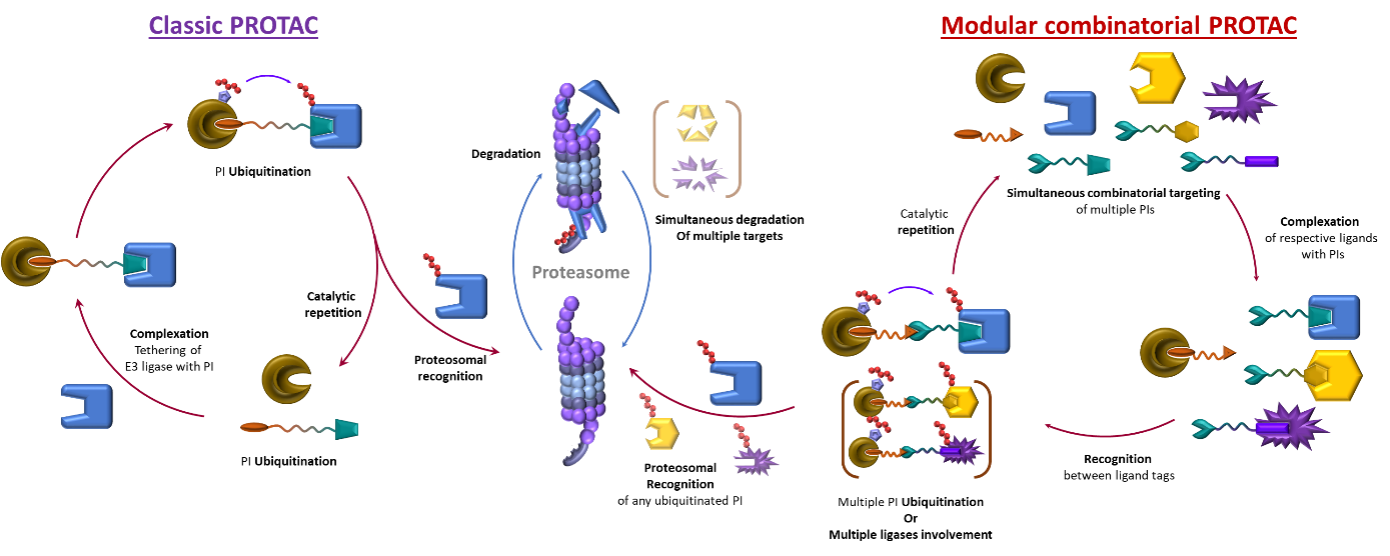
Figure 3: Left: Single target, single ligase mode of action of classic Protac; Right: Combinatorial multitargeting mode of action of Dynamic combinatorial Protac.
Using Copper as a supramolecular glue for specific and targeted PROTACs action
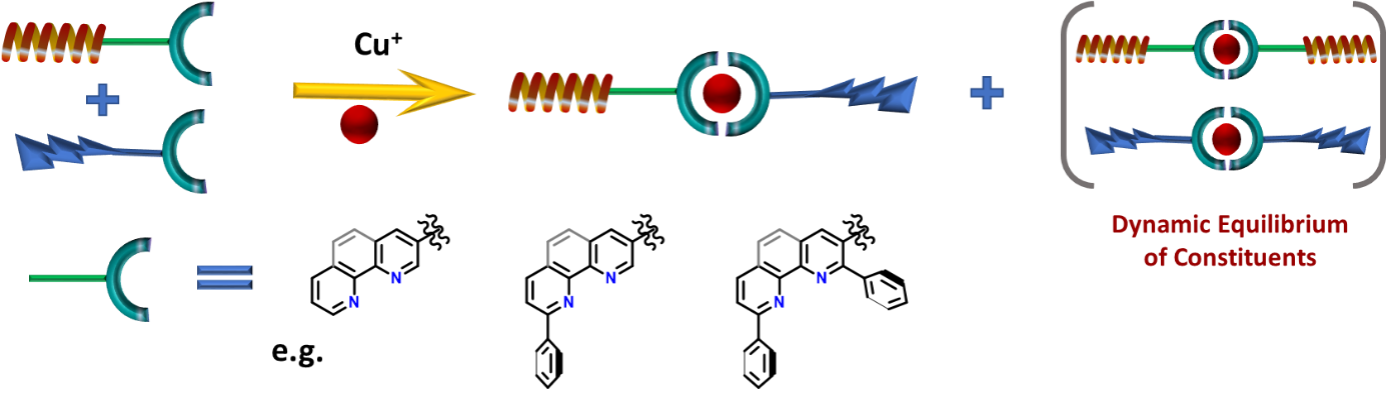
Figure 4: Illustration of the use of metal coordination as a dynamic linking motive
Advantageously, there are already known convenient, dynamic motives such as metal-ligand coordination (e.g. Copper), hydrogen bonding or other supramolecular interactions, which are suitable for this project's scope.
The primary focus will be on Copper, a biogenic metal naturally present in cells. Copper complexes are also labile, meaning they undergo rapid ligands exchange. This process is tuneable by the design of organic ligands and ensures the statistical formation of heterobifunctional active species and catalytic mode of action.7
The project will begin with synthesising model ligands based on phenanthroline and pyridines, widely used ligands with a tunable affinity towards Cu through substitution. After that, their equilibrium distribution and association constants will be studied. The best matching motives will be finally tested in cell cultures. For a proof-of-concept stage, in-vivo studies will start with fluorescent dyes instead of active moieties. This will allow us to study the distribution of ligands inside the cell and confirm their dynamic association under biological conditions. Finally, the two sets of compatible bioactive ligands will be prepared and tested for protein degradation.
The metal for proof of concept was chosen Copper for the abovementioned reasons – advantageous dynamic coordination, bioavailability and clinical relevance. However, one can easily envisage other first-row transition metals, such as iron, as another possible target.
Literature:
1) Zhong Y, Chi F, Wu H, Liu Y, Xie Z, Huang W, Shi W, Qian H. Emerging targeted protein degradation tools for innovative drug discovery: From classical PROTACs to the novel and beyond. Eur J Med Chem. 2022, 231, 114142. doi: 10.1016/j.ejmech.2022.114142
2) Qi SM, Dong J, Xu ZY, Cheng XD, Zhang WD, Qin JJ. PROTAC: An Effective Targeted Protein Degradation Strategy for Cancer Therapy. Front Pharmacol. 2021, 12, 692574. doi: 10.3389/fphar.2021.692574.
3) Li, X., Song, Y. Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy. J Hematol Oncol 13, 50 (2020). https://doi.org/10.1186/s13045-020-00885-3
4) Philipp M. Cromm, Craig M. Crews and Hilmar Weinmann, Chapter 1:PROTAC-mediated Target Degradation: A Paradigm Changer in Drug Discovery? , in Protein Degradation with New Chemical Modalities: Successful Strategies in Drug Discovery and Chemical Biology, 2020, pp. 1-13 DOI: 10.1039/9781839160691-00001
5) Cecchini C, Pannilunghi S, Tardy S, Scapozza L. From Conception to Development: Investigating PROTACs Features for Improved Cell Permeability and Successful Protein Degradation. Front Chem. 2021 Apr 20;9:672267. doi: 10.3389/fchem.2021.672267. PMID: 33959589; PMCID: PMC8093871.
6) Xin Han, Lijie Zhao, Weiguo Xiang, Chong Qin, Bukeyan Miao, Tianfeng Xu, Mi Wang, Chao-Yie Yang, Krishnapriya Chinnaswamy, Jeanne Stuckey, and Shaomeng Wang Discovery of Highly Potent and Efficient PROTAC Degraders of Androgen Receptor (AR) by Employing Weak Binding Affinity VHL E3 Ligase Ligands; Journal of Medicinal Chemistry 2019 62 (24), 11218-11231; DOI: 10.1021/acs.jmedchem.9b01393
7) Satoshi Umeki, Shoko Kume, and Hiroshi Nishihara Switching of Molecular Insertion in a Cyclic Molecule via Photo- and Thermal Isomerization Inorganic Chemistry 2011 50 (11), 4925-4933 DOI: 10.1021/ic200145h
Preparation and activity study for Organoruthenium electrocatalysts for water and ammonia oxidation – towards sustainable Hydrogen production
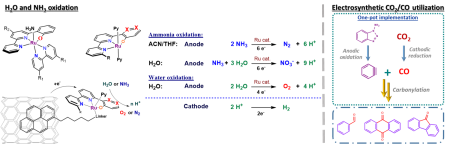
Electrochemical Oxidation:
It is more and more evident that humankind has to develop new cleaner energy sources to replace obsolete fossil fuels. Many see hydrogen as such fuel for the future. However, its volatility, sensitivity, high liquefaction and transportation costs hinder its wide-scale use. These obstacles could be solved by developing an electrochemical cell that liberates hydrogen from a possibly benign compound for direct on-site use. Below, two processes (Water and Ammonia oxidation, Figure 2)) are introduced to illustrate this concept. The significant advantage here is their complementarity, meaning that the mechanistic rationale and catalysts from one process are directly transferable to the other one
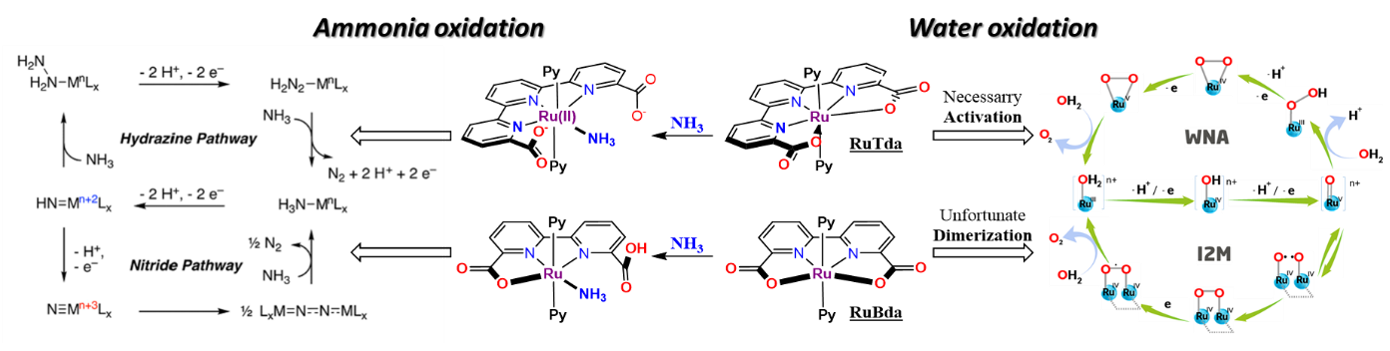
Figure 1: Comparison of catalytic mechanisms of RuTda (monomolecular) and RuBda (dimeric); Left: Ammonia oxidation; Right: Water oxidation
Water oxidation catalysis (WOC)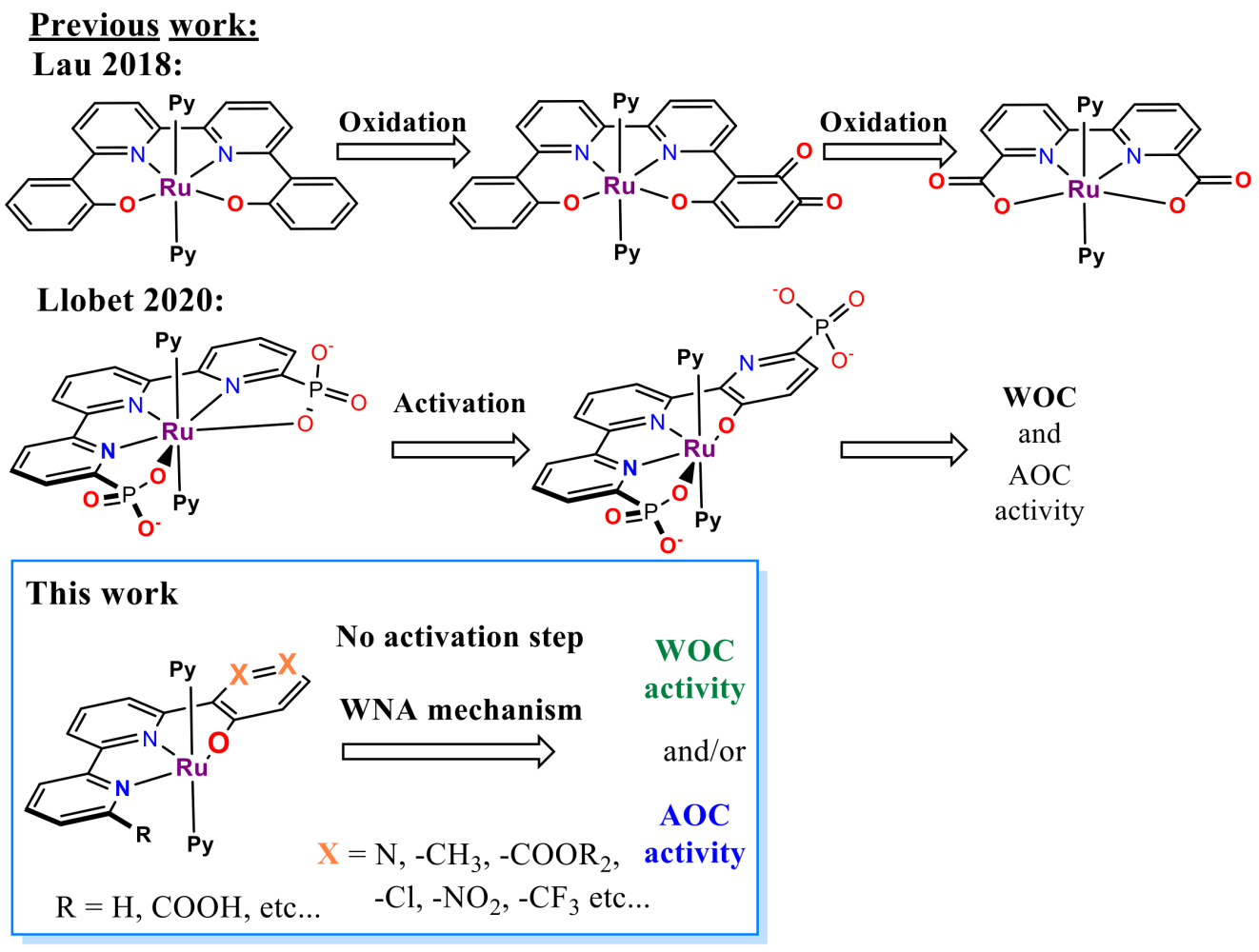
The most pursued concept relies naturally on H2O combined with a water oxidation catalyst. Water oxidation has been a holy grail of green renewable energy since the term was coined. The sheer abundance and complete harmlessness make water a dream source of energy. However, even after years of research, there is no economically viable commercial utilisation. In this regard, the closest to an application are ruthenium-based electrocatalysts with their low overpotentials, comfortable working window in a whole pH scale and relatively high stability.[1] In contrast, first-row transition metal-based WOC catalysts suffer from their reliance on extreme pH and high overpotentials while displaying low life duration (Low TOFs and TONs).[1] From an economic point of view, if ruthenium catalysts are stable in the long term, the initial high investment can be balanced with a sustained, steady and long-lasting return.
The field of ruthenium water oxidation catalysts has seen striking development in recent years with the rise of two prominent families - the RuBda[2] (Bda = 2,2’-Bipyridine-6,6’-dicarboxylic acid) and RuTda[3] (Tda = 2,2':6',2''-Terpyridine]-6,6''-dicarboxylic acid) (Figure 2).
The literature sources show already a wide range of studies dealing with derivatisation, surface anchoring and applications.[1] However, each of these families still suffers from its own critical weaknesses preventing their practical use. For example, RuTda, the most promising catalyst for application, as it works through monomolecular water nucleophilic attack (WNA) mechanism (Figure 2), needs a bulk electrolytic activation under strongly basic conditions to reach its peak performance.[3] In comparison, RuBda does not need any activation but works through a dimeric (Interaction of two M-O units – I2M) mechanism, which renders this catalyst unsuitable for surface anchoring and consequently unsuitable for practical applications. The surface anchoring increases the active concentration of the catalyst in the diffusion layer at the electrode and improves electron transfer and the overall robustness of the electrochemical set-up.
It was shown that the structural analogue of RuTda, the RutPa undergoes partial oxidation under the catalytic conditions giving a new pyridine-3-oxo species (Figure 3), which is assumed to be the actual active catalyst.[4] Building upon the structural similarities, introducing the hydroxyl group into the terpyridine offers good chances to eliminate the independent activation step.
Ammonia oxidation catalysis (AOC)
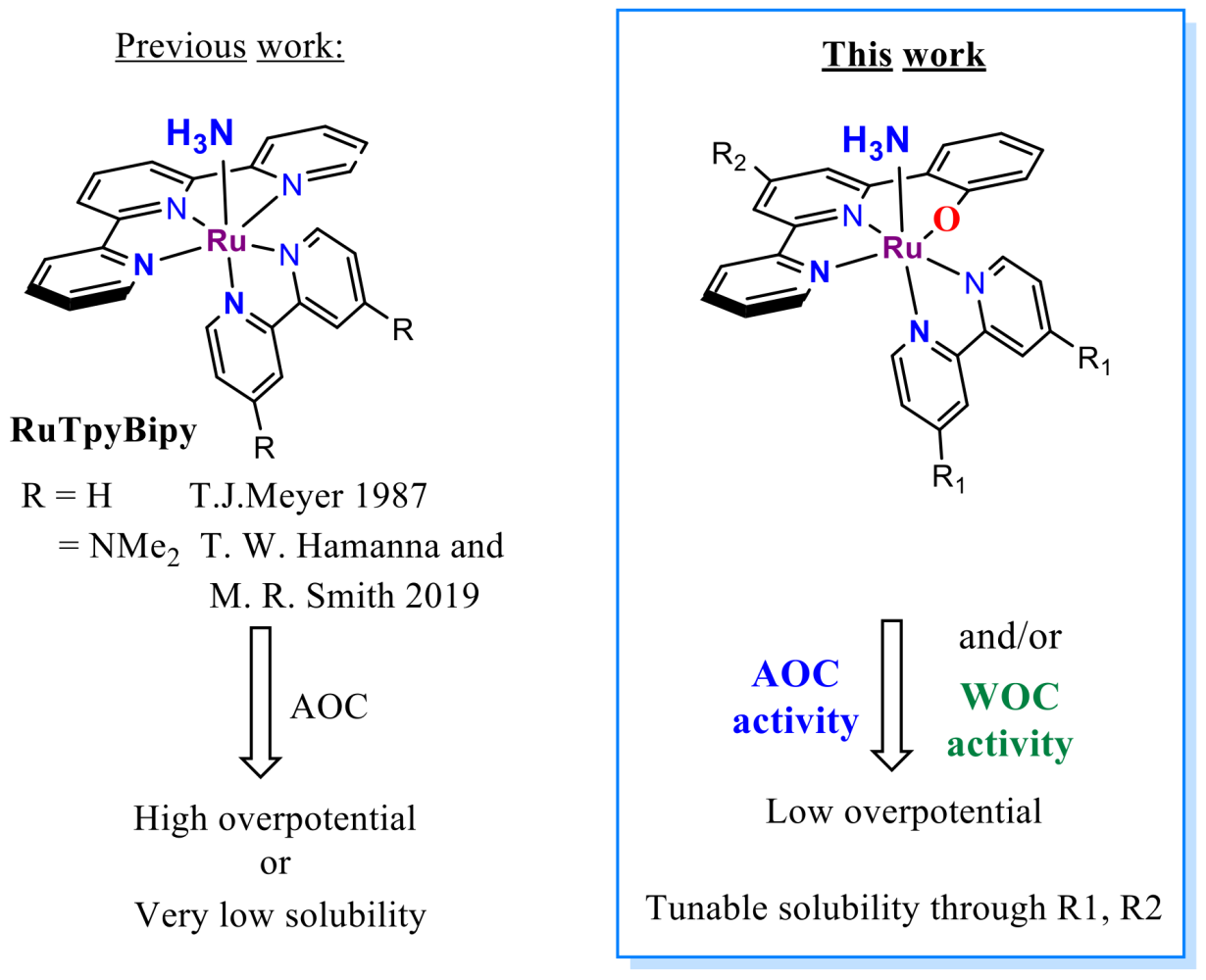
As an alternative to water, ammonia has risen in the last two years as a challenger for future hydrogen source. Its advantages are low thermodynamic constraints for electrocatalytic oxidation (NH3 -0.092 V vs H2O 1.23V vs NHE), the high energy density of liquid ammonia (1.5x of liquid H2) and existing worldwide processing infrastructure[5]. Low oxidative potential means lower operational costs and cheaper devices. Also, the existing infrastructure indicates that with any future progress in NH3 synthesis, this approach can quickly become technologically feasible. Coincidentally, it has been shown by several studies that the same complexes active in WOC can also be applied in AOC, meaning that discoveries from one process can be directly applied to the other and vice versa.
The most notable discoveries in this field, both from 2019, are Hamannna and Smith's improvement of the old RuTerpyBipy[6][7] motive (RuTerpy[Bipy(NMe2)2]) and Nishibayashi's RuBda study[8] (Figure 4). The RuBda again works through a disadvantageous bimolecular mechanism (Figure 2) relying on the dimerisation of Ru-bound nitride units, thus effectively thwarting further practical applications.
In comparison, Hamanna's and Smith's RuTerpy[Bpy(NMe2)2] (Figure 4) catalyst works through an advantageous monomolecular mechanism relying on the nucleophilic attack of ammonia to Ru-bound imine. However, the –NMe2 used to shift electrochemical potentials significantly reduced the solubility of the complex to the point where it prevented any further development. [6] The aim here is, again, through the introduction of the hydroxyl group keep the low overpotential[9] while maintaining the good solubility of the catalyst. Furthermore, it was shown that the exchange of N for –O- in Ru(Bpy)3 type complex leads to a favourable cathodic shift for more than 0.5 V at RuII/III transition. [9]
1) Ligands and the Complexes
At first, two distinguished families of ligands and complexes with coordinated aryl/pyridyl oxide will be synthesised and tested in WOC and AOR accordingly. (Figure 5)
The first catalyst family will be built over the RuTerpyBipy motive using the bipyridine-phenoxide (BPPO) (1, Figure 4 and 5b) ligand bearing no auxiliary substituents (R1 = -H, Figure 5b). Later the substitution on BPPO or bipyridine outer rim can fine-tune its physicochemical properties (R1, R2 Figure 4). Although the RuTerpyBpy design mainly targets AOC reactivity, it also bears considerable potential for WOC.[10]
In addition to hydroxyl, the second family will also have the positions 3, 4- (in regard to hydroxyl, Figures 3 and 2 in Figure 5b) occupied either with heteroatoms, decorated with functional groups (e.g. COOR, CF3 etc.) or a combination of both. The inherent properties of Ruthenium ensure that the hydroxyl coordinates to the metal centre achieving N2O2 coordination vicinity. The ability of pyridine to rotate[4] provides an opportunity for auto-corrective hydroxyl coordination in the case of the nitrogen coordinates first (Figure 3). Furthermore, the additional substituents will protect the aromatic core from further oxidation, thus preventing the decomposition described by Lau.[11] The resemblance to RuTda predetermines this type of complex for WOC; however, the preliminary results also give confidence for its complementary activity in AOC.
2) Electrochemical characterisation, mechanistic studies and catalytic performance assessment
After the synthesis, all the compounds will be subjected to electrochemical analysis (CV, DPV, CPE, etc.) and assessment. The elegance of the electrochemical methods is in their experimental simplicity while still being highly informative. By measuring CVs at different scan rates, concentrations or variations in other conditions, it is possible to get highly precise information about TON and TOF as well as other kinetic and mechanistic data. Later, through the bulk electrolysis, it will be possible to assess the stability of our catalyst under working conditions as well as to quantify the amount of produced gasses (O2 or N2 and H2) via analysis of reaction headspace with gas chromatography or other established analytic method (e.g. Clark electrode).
3) Enhancement and tuning of our catalysts
The described design of the catalysts allows simple modification of the ligands and the metal core for alternative catalytic processes. Despite many possibilities, the most thrilling one is the addition of the pyrene moiety. This will allow us to anchor our catalysts through π-π stacking on the graphitic surfaces and, consequently, onto the electrodes through the drop-casting deposition. (Figure 6). This simple adjustment can enhance the electron transfer from the electrode to the catalyst, increase its effective concentration in the active diffusion layer and lead to stable, reusable electrodes for long-term applications. [12]
Further options include introducing other solubilising and electron-donating/withdrawing groups to tune the physicochemical properties of the complexes. Lastly, the ligand invites for complexation with different metal cations such as Os, Ir or first-row transition metals such as Mn, Fe. This variability can open the door to new processes and serve as both a backup strategy and added valuation for the ligands.
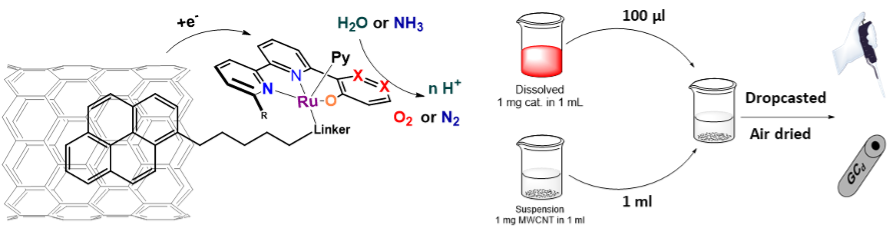
Figure 4: Illustration of the catalyst anchoring via π-π stacking and visualisation of the drop-casting process
[1] R. Matheu, P. Garrido-Barros, M. Gil-Sepulcre, M. Z. Ertem, X. Sala, C. Gimbert-Suriñach, A. Llobet, Nat. Rev. Chem. 2019, 3, 331–341.
[2] L. Duan, F. Bozoglian, S. Mandal, B. Stewart, T. Privalov, A. Llobet, L. Sun, Nat. Chem. 2012, 4, 418–423.
[3] R. Matheu, M. Z. Ertem, J. Benet-Buchholz, E. Coronado, V. S. Batista, X. Sala, A. Llobet, J. Am. Chem. Soc. 2015, 137, 10786–10795.
[4] N. Vereshchuk, R. Matheu, J. Benet-Buchholz, M. Pipelier, J. Lebreton, D. Dubreuil, A. Tessier, C. Gimbert-Suriñach, M. Z. Ertem, A. Llobet, J. Am. Chem. Soc. 2020, 142, 5068–5077.
[5] N. M. Adli, H. Zhang, S. Mukherjee, G. Wu, J. Electrochem. Soc. 2018, 165, J3130–J3147.
[6] F. Habibzadeh, S. L. Miller, T. W. Hamann, M. R. Smith, Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 2849–2853.
[7] M. S. Thompson, T. J. Meyer, J. Am. Chem. Soc. 1981, 103, 5577–5579.
[8] K. Nakajima, H. Toda, K. Sakata, Y. Nishibayashi, Nat. Chem. 2019, 11, 702–709.
[9] B. M. Holligan, J. C. Jeffery, M. K. Norgett, E. Schatz, M. D. Ward, J. Chem. Soc. Dalt. Trans. 1992, 3345–3351.
[10] R. Matheu, M. Z. Ertem, C. Gimbert-Suriñach, J. Benet-Buchholz, X. Sala, A. Llobet, ACS Catal. 2017, 7, 6525–6532.
[11] Y. Liu, G. Chen, S. M. Yiu, C. Y. Wong, T. C. Lau, ChemCatChem 2018, 10, 501–504.
[12] J. Creus, R. Matheu, I. Peñafiel, D. Moonshiram, P. Blondeau, J. Benet-Buchholz, J. García-Antón, X. Sala, C. Godard, A. Llobet, Angew. Chemie - Int. Ed. 2016, 55, 15382–15386.
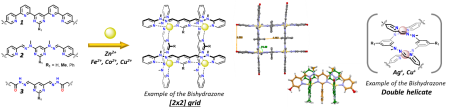
Supramolecular grids as building blocks in responsive self-healing polymers
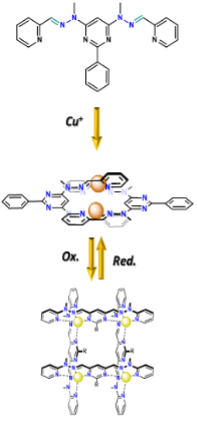 Grids – building blocks for Smart Materials
Grids – building blocks for Smart Materials
In the frame of this project, new stimuli-responsive materials will be developed via the incorporation of metaloresponsive ligands and grids into the polymeric matrix.[1]
As shown in Figure 1, the grid's ligands can adopt three different states: metal-free native conformation, double helicate (Cu+, Ag+) or grid geometry (Zn2+, Fe2+, Cu2+) according to the presence and coordination preferences of metal cations (Figure 2a).[2]
Two possible strategies will be tested to incorporate ligands into the polymeric matrix. The first will be through the preparation of shorter fragments bearing the grid's ligands on their ends (Figure 2b, Left).[1] Such fragments are usually viscous liquids, which will crosslink after the addition of cations and form a solid material. The features of such a composite will depend on the type of cation and polymeric fragment. The second approach will incorporate ligands inside the polymeric chain through the co-polymerisation of peripheral groups with isocyanate ends of polymeric fragments (Figure 2b, Right).[1]
Besides the metalo-responsive behaviour, the material should also display self-healing properties, thanks to the reversible assembly of the grids and helicates (Figure 2c).
Switching between three states of the ligands will then directly affect the properties of the bulk material. These different nanoscopic changes/movements should translate into macroscopic changes of shape, density or conductivity. Furthermore, it should be possible to reversibly control these changes in a stimuli-responsive manner through the changes in the oxidation state of the metal cation (Cu+/2+). The supramolecular extension of this methodology entails applications like responsive hydrogels, artificial muscles or responsive self-assembling polymer particles.[3,1]
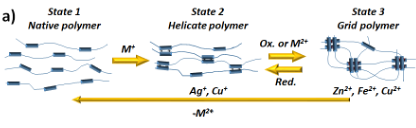
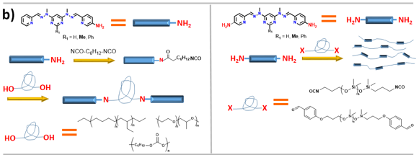
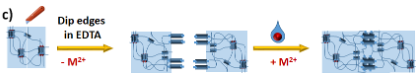
Figure 2: a) Transitions between free ligand, double helix and grid inside the polymeric matrix; b) The synthesis of polymers with incorporated ligands; c) Illustration of the self-healing process
[1] N. Roy, B. Bruchmann, J. M. Lehn, Chem. Soc. Rev. 2015, 44, 3786–3807
[2] A. M. Stadler, C. Burg, J. Ramírez, J. M. Lehn, Chem. Commun. 2013, 49, 5733–5735.
[3] J. G. Hardy, X. Y. Cao, J. Harrowfield, J. M. Lehn, New J. Chem. 2012, 36, 668–673.
[urlnadstranka] => [ogobrazek] => [pozadi] => [iduzel] => 67575 [canonical_url] => [skupina_www] => Array ( ) [url] => /vyzkum/koordinacni-chemie/prace/grids_polymers [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) ) [67540] => stdClass Object ( [nazev] => [seo_title] => Self-assembling recyclable surface platforms for catalysis and sensing based on supramolecular grids [seo_desc] => [autor] => [autor_email] => [obsah] =>
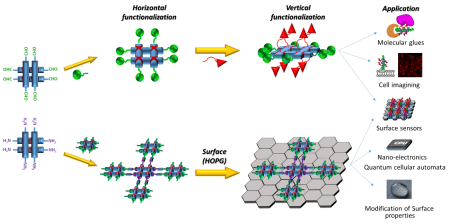
Self-assembling recyclable surface platforms for catalysis and sensing based on supramolecular grids
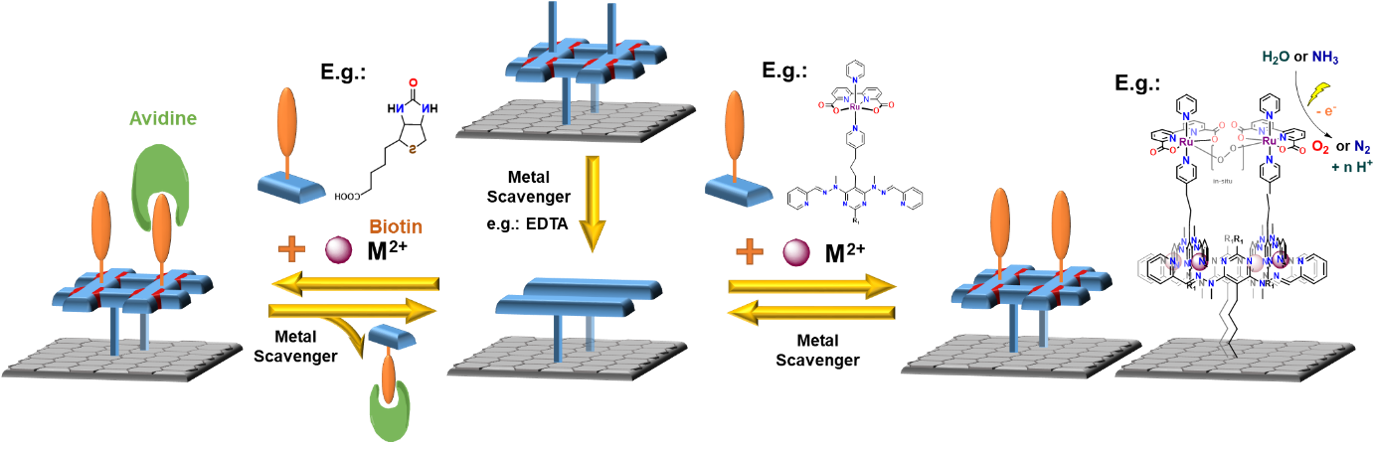
Figure 1: Assembly of surface anchored heterostranded grid and depiction of heterogenous molecular electrode with RuBda for electrochemical water oxidation
Introduction and project summary
Much effort in the field of surface engineering is devoted to the assembly of regular arrays. However, many applications do not require extensive 2D networks. Already a simple anchoring to the surface can dramatically improve different catalytic properties such as electron transfer in electrocatalysis or help in artificial sensors or scavengers for biochemistry-related projects (Biotin-based scavenging).
This project aims to prepare grid-based surface-bound platforms, which will be reversibly self-assembling/disassembling, responsive to external stimuli and customisable for different applications.
As depicted in Figure 1, anchoring a grid to a surface followed by metal removal (EDTA, cryptands) will disassemble the grid structure. This will leave behind a set of anchored ligands correctly pre-placed for a repetitive re-assembly of the grid moiety. Subsequent addition of new metal cations and readily functionalised grid ligands will lead to a new heterostranded grid formation. Due to the presence of permanently anchored ligands, it will be possible to repeat this process anytime when the functionality needs to be restored or changed, thus creating reusable/recyclable surface support for applications in different fields. (Figure 1)
Figure 1 also depicts an example of a fabrication process of a molecular electrode for heterogeneous electrocatalysis using an anchored grid functionalised with a current "state of the art" water oxidation catalysts - RuBda.[1] The catalyst is known to dimerise during the key step of its catalytic cycle. In this case, the classic individual anchoring drastically reduces its activity due to the hindrance of this process. The installation of two RuBda moieties in a well-defined (tuneable) distance within the grid scaffold should enable their dimerisation and unlock the catalysts for practical applications. The repeatable assembly will allow the recycling of the grid platform in the case of catalysts degradation, leaching or its exchange for a different catalyst.
Methodology
In the scope of this work, the students will first prepare and study the assembly and disassembly of the simple grids in solution. Afterwards, they will try to anchor the grids on different surfaces and study the assembly/disassembly process on the surface using spectroscopic and electrochemical techniques[2] (Figures 2-4).
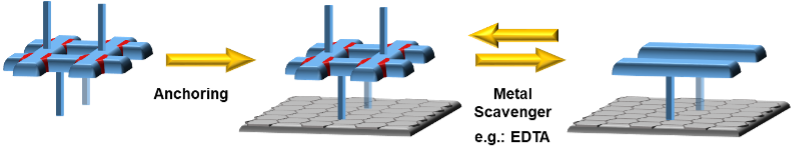
Figure 2: The proof-of-concept assembly and disassembly of the basic grid motive.
Visualisation of the whole assembly/disassembly process will also be done electrochemically using differing CV responses during the different states of assembly (Figure 3). Using different cations, one will be able to obtain different electrochemical traces and thus confirm the re-assembly of the coordination complex.
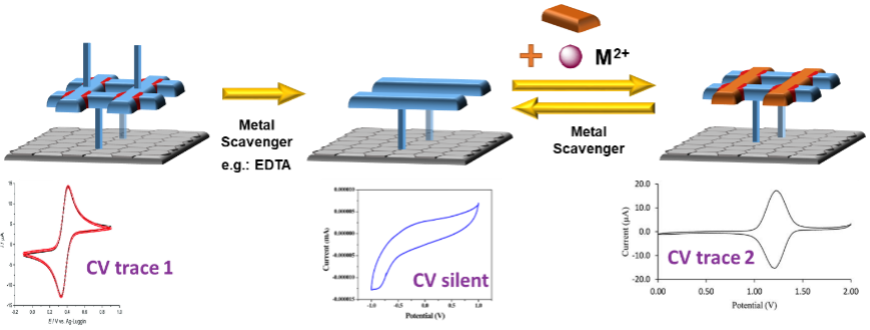
Figure 3: The use of different redox-active metals to distinguish the beginning and end states of the grid
When control over the whole process is established, we will use ligands bearing redox-active groups to prepare more complex and functional systems. In addition to using different metal cations, the ligand exchange will prove that the newly re-assembled complex contains new ligands and not only cations and will also open a path for more practical applications of the system (Figure 4).
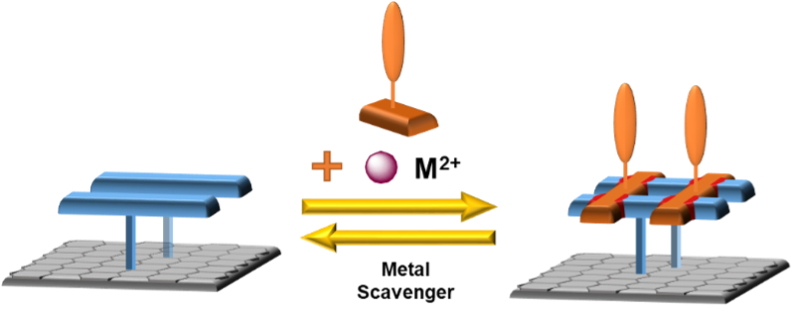
Figure 4: The use of different redox-active ligands to generate functional grid assemblies
Applications:
RuBda for electrocatalysis
In the final stage, the platform will be assembled with ligands bearing RuBda water oxidising electrocatalyst (Figure 5). The RuBda is chosen because its mechanism of function requires the formation of a (-O-O- bridged) dimer. [1] Therefore, the performance of classically covalently anchored single molecule RuBda on the surface is drastically inferior to its performance in the solution. [3] In this regard, the highly regular spatial arrangement of the grids should hold two (or more) RuBda molecules at distances favourable for dimer formation and enhance its performance in the scope of heterogenous molecular anodes in electrochemical water or ammonia oxidation catalysis. These processes are of utmost importance for our efforts to achieve a carbon-free and sustainable energy cycle.
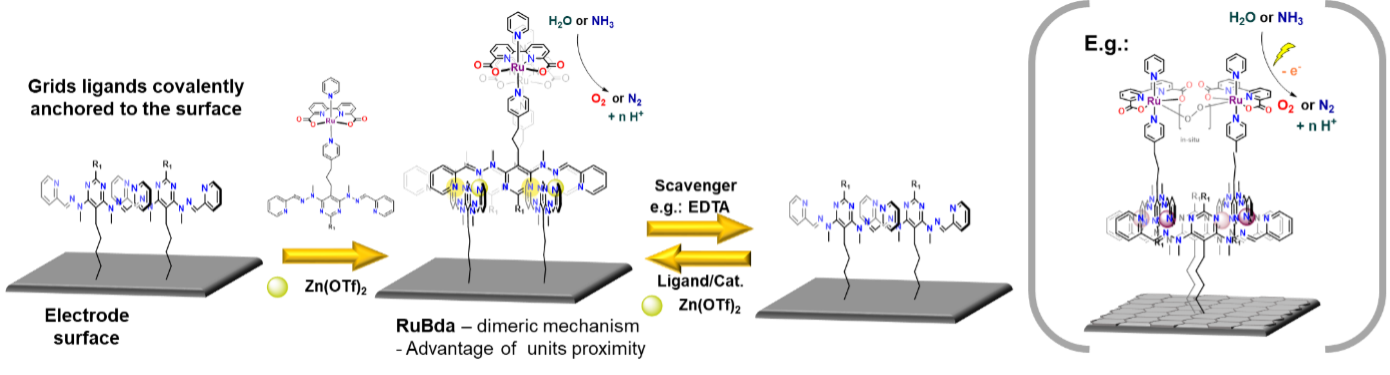
Figure 5: Functionalisation with RuBda for effective and practical (electro)catalysis
Sensors/Biochemical pull down (Far, far away future)
The same concept could also be used for biochemically relevant pull-down techniques or custom-made sensors. [4] (Figure 6)
[1] L. Duan, F. Bozoglian, S. Mandal, B. Stewart, T. Privalov, A. Llobet, L. Sun, Nat. Chem. 2012, 4, 418–423.
[2] N. Elgrishi, K. J. Rountree, B. D. McCarthy, E. S. Rountree, T. T. Eisenhart, J. L. Dempsey Journal of Chemical Education 2018, 95 (2), 197-206
[3] R. Matheu, L. Francàs, P. Chernev, M. Z. Ertem, V. Batista, M. Haumann, X. Sala, A. Llobet ACS Catalysis 2015, 5 (6), 3422-3429
[4] Mochizuki Y, Kohno F, Nishigaki K, Nemoto N. Anal Biochem, 2013, 434, 93-5
Figure 6: Biotin scavanging system used
in biochemistry for protein pulldown.
[urlnadstranka] => [ogobrazek] => [pozadi] => [iduzel] => 67540 [canonical_url] => [skupina_www] => Array ( ) [url] => /vyzkum/koordinacni-chemie/prace/grids_surface [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) ) ) [iduzel] => 38838 [canonical_url] => [skupina_www] => Array ( ) [url] => /vyzkum/koordinacni-chemie/prace [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) ) [29407] => stdClass Object ( [nazev] => Výzkum [seo_title] => Výzkum [seo_desc] => [autor] => [autor_email] => [obsah] =>
Řešená problematika
Elektrochemie karbenových komplexů chromu a železa
Součástí tohoto projektu je sledování redoxních vlastností v závislosti na změnách struktury studovaných molekul. K tomuto studiu používáme jak klasické elektrochemické metody (např. cyklickou voltametrii), tak spektroelektrochemickém metody, které kombinují elektrochemická a spektroskopická měření (UV-Vis, IR a MS). Sledované závěry jsou podpořeny výpočty rozložení a energie molekulových orbitalů, které se podílejí na oxidaci či redukci. Nedílnou součástí je také teoretická predikce naměřených spekter. Nejčastěji k výše uvedeným výpočtům používáme DFT. Na této problematice spolupracujeme s kolegy z Ústavu organické chemie VŠCHT a Ústavu fyzikální chemie JH AV ČR.
Syntéza a charakteristika komplexů s ligandy typu Schiffových bazí odvozených od vitamínů B
V rámci této problematiky se zaměřujeme především na syntézu sloučenin, které jsou blízké molekulám v lidském těle (deriváty vitamínu B). Se syntetickou prací je zejména spojeno studium vlivu reakčních podmínek na vznik požadovaných koordinačních sloučenin, zejména vlivu centrálního kovu na reaktivitu ligandů. Průběh interakce kovu s ligandy sledujeme spektroskopickými metodami, především UV-Vis spektroskopií, a také elektrochemickými metodami (např. cyklickou voltametrií). Pomocí těchto metod lze sledovat tvorbu a stabilitu těchto komplexů a změny vlastností kovů i ligandů po komplexaci.
[urlnadstranka] => [obrazek] => [ogobrazek] => [pozadi] => [poduzel] => Array ( ) [iduzel] => 29407 [canonical_url] => [skupina_www] => Array ( ) [url] => /vyzkum/koordinacni-chemie/vyzkum [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) ) [29082] => stdClass Object ( [nazev] => Publikace [seo_title] => Publikace [seo_desc] => [autor] => [autor_email] => [obsah] =>
2024
- Sumner, E.; Pižl, M.; McQuaid, K. T.; Hartl, F. Nitrile Substituents at the Conjugated Dipyridophenazine Moiety as Infrared Redox Markers in Electrochemically Reduced Heteroleptic Ru(II) Polypyridyl Complexes. Inorganic Chemistry 2024, 63 (5), 2460-2469.
2023
- Štefanková, D.; Skrbek, K.; Pižl, M.; Bartůněk, V. Nano and mesosized selenium and its synthesis using the ascorbic acid route. Journal of Non-Crystalline Solids 2023, 616, 122462.
2023
- Štefanková, D.; Skrbek, K.; Pižl, M.; Bartůněk, V. Nano and mesosized selenium and its synthesis using the ascorbic acid route. Journal of Non-Crystalline Solids 2023, 616, 122462.
- Kearney, L.; Brandon, M. P.; Coleman, A.; Chippindale, A. M.; Hartl, F.; Lalrempuia, R.; Pižl, M.; Pryce, M. T. Ligand-Structure Effects on N-Heterocyclic Carbene Rhenium Photo- and Electrocatalysts of CO2 Reduction. Molecules 2023, 28 (10), 4149.
2022
- Pižl, M.; Hunter, B. M.; Sazanovich, I. V.; Towrie, M.; Gray, H. B.; Záliš, S.; Vlček, A., Excitation-Wavelength-Dependent Photophysics of d8d8 Di-isocyanide Complexes. Inorganic Chemistry 2022, 61 (6), 2745-2759
2021
- Taylor, J. O.; Pižl, M.; Kloz, M.; Rebarz, M.; McCusker, C. E.; McCusker, J. K.; Záliš, S.; Hartl, F.; Vlček, A., Optical and Infrared Spectroelectrochemical Studies of CN-Substituted Bipyridyl Complexes of Ruthenium(II).Inorganic Chemistry 2021, 60 (6), 3514-3523
2020
- Sondermann, C.; Pižl, M.; Paretzki, A.; Feil, C.; Ringenberg, M. R.; Záliš, S.; Kaim, W., Analysis of a Diimine-Organonickel Redox Series. European Journal of Inorganic Chemistry 2020, 2020 (31), 3010-3015.
- Pižl, M.; Picchiotti, A.; Rebarz, M.; Lenngren, N.; Yingliang, L.; Záliš, S.; Kloz, M.; Vlček, A., Time-Resolved Femtosecond Stimulated Raman Spectra and DFT Anharmonic Vibrational Analysis of an Electronically Excited Rhenium Photosensitizer. The Journal of Physical Chemistry A 2020, 124 (7), 1253-1265.
- Guricová, M.; Tobrman, T.; Pižl, M.; Žižková, S.; Hoskovcová, I.; Dvořák, D., Synthesis, characterisation and electrochemical properties of Cr(0) aminocarbene complexes containing condensed heteroaromatic moiety. J Organomet Chem 2020, 905, 121023.
2019
- Takematsu, K.; Pospíšil, P.; Pižl, M.; Towrie, M.; Heyda, J.; Záliš, S.; Kaiser, J. T.; Winkler, J. R.; Gray, H. B.; Vlček, A., Hole Hopping Across a Protein–Protein Interface. The Journal of Physical Chemistry B 2019, 123 (7), 1578-1591.
- Zelenka, J.; Svobodová, E.; Tarábek, J.; Hoskovcová, I.; Boguschová, V.; Bailly, S.; Sikorski, M.; Roithová, J.; Cibulka, R., Combining Flavin Photocatalysis and Organocatalysis: Metal-Free Aerobic Oxidation of Unactivated Benzylic Substrates. Organic Letters 2019, 21 (1), 114-119.
2018
- Mojr, V.; Pitrová, G.; Straková, K.; Prukała, D.; Brazevic, S.; Svobodová, E.; Hoskovcová, I.; Burdziński, G.; Slanina, T.; Sikorski, M.; Cibulka, R., Flavin Photocatalysts for Visible-Light [2+2] Cycloadditions: Structure, Reactivity and Reaction Mechanism. ChemCatChem 2018, 10 (4), 849-858.
- Chen, L.; Lim, K. J. C.; Babra, T. S.; Taylor, J. O.; Pižl, M.; Evans, R.; Chippindale, A. M.; Hartl, F.; Colquhoun, H. M.; Greenland, B. W. A macrocyclic receptor containing two viologen species connected by conjugated terphenyl groups. Organic & Biomolecular Chemistry 2018, 16 (27), 5006-5015
- Murašková, V.; Dušek, M.; Buryi, M.; Laguta, V.; Huber, Š.; Sedmidubský, D. Synthesis, characterization and X-ray crystal structure of an iron(III) complex of a tripodal pyridoxal Schiff base ligand: effects of positional disorder on its magnetic properties, Transit. Met. Chem. 2018, 43, 605-619
- Pižl, M.; Jankovský, O.; Guricová, M.; Hoskovcová, I.; Sedmidubský, D.; Bartůněk, V. Mixed Yttrium–Ytterbium–Erbium Schiff Base Complex as a Model Precursor for Mixed Nanosized Rare Earths Oxides. Journal of Cluster Science 2018, 29 (4), 549-553
- Guricová, M.; Pižl, M.; Smékal, Z.; Nádherný, L.; Čejka, J.; Eigner, V.; Hoskovcová, I., Template synthesis and structure of Co(II), Ni(II), and Cu(II) complexes with pyridoxilydenetaurinate Schiff base ligand. Inorganica Chimica Acta 2018, 477, 248-256.
2017
- Pižl, M.; Hunter, B. M.; Greetham, G. M.; Towrie, M.; Záliš, S.; Gray, H. B.; Vlček, A., Ultrafast Wiggling and Jiggling: Ir2(1,8-diisocyanomenthane)42+. The Journal of Physical Chemistry A 2017, 121 (48), 9275-9283.
- Murašková, V.; Szabó, N.; Pižl, M.; Hoskovcová, I.; Dušek, M.; Huber, Š.; Sedmidubský, D., Self assembly of dialkoxo bridged dinuclear Fe(III) complex of pyridoxal Schiff base with CC bond formation – Structure, spectral and magnetic properties. Inorganica Chimica Acta 2017, 461, 111-119.
- Pižl, M.; Jankovský, O.; Ulbrich, P.; Szabó, N.; Hoskovcová, I.; Sedmidubský, D.; Bartůněk, V., Facile preparation of nanosized yttrium oxide by the thermal decomposition of amorphous Schiff base yttrium complex precursor. Journal of Organometallic Chemistry 2017, 830, 146-149.
2016
- Langmaier, J.; Pižl, M.; Samec, Z.; Záliš, S.; Extreme Basicity of Biguanide Drugs in Aqueous Solutions: Ion Transfer Voltammetry and DFT Calculations. The Journal of Physical Chemistry A 2016, 120 (37), 7344-7350.
- Váňová, H.; Tobrman, T.; Hoskovcová, I.; Dvořák, D.; Modular Synthesis of Fischer Biscarbene Complexes of Chromium. Organometallics, 2016, 35(17), 2999-3006
2015
- Metelková, R.; Hoskovcová, I.; Polášek, M.; Urban, J.; David, T.; Ludvík, J.; Stereoisomeric products of electrochemical reduction of heterocyclic Fischer aminocarbene Cr(0) complexes. Development of the electrochemistry-mass spectrometry tandem approach using biphasic (acetonitrile-hexane) preparative electrolysis. Electrochimica Acta 2015, 162, 17-23
2014
- Kvapilová, H.; Hoskovcová, I.; Ludvík, J.; Záliš, S.; Theoretical Predictions of Redox Potentials of Fisher-Type Chromium Aminocarbene Complexes. Organometalics, 2014, 33, 4964-4972
- Kvapilová, H.; Eigner, V.; Hoskovcová, I.; Tobrman, T.; Čejka, J.; Záliš, S.; Structural flexibility of 2-hetaryl chromium aminocarbene complexes: Experimental and theoretical evidence. Inorganica Chimica Acta 2014, 421, 439-445
Kapitola ve sborníku
- ELECTROCHEMISTRY OF FISCHER AMINOCARBENE
COMPLEXES: EFFECTS OF STRUCTURE ON REDOX
PROPERTIES, ELECTRON DISTRIBUTION, AND
REACTION MECHANISMS
Jiří Ludvík, Irena Hoskovcová
Chapter 48, p.653-665 in:
Advances in Organometallic Chemistry and Catalysis: The Silver/Gold Jubilee International Conference on Organometallic Chemistry Celebratory Book,
First Edition. Edited by Armando J. L. Pombeiro.
© 2014 John Wiley & Sons, Inc. Published 2014 by John Wiley & Sons, Inc
2022
- Holub, J.; 8th EuChemS Chemistry Congress, Lisbon (PT), 28.8.-1.9.2022
- Pižl, M.; Sumner, E.; Jiříčková, A.; Kulagová, A.; Hartl, F.; 44th International Conference on Coordination Chemistry, Rimini (I), 28.8.-2.9.2022
- Kulagová, A. Svoboda, D.; Kotoučová, H.; Hoskovcová, I.; ISE Regional Meeting, Prague (CZ), 15.-19.8.2022
2019
- Pižl, M.; Fuse, M.; Taylor, J. O.; Donaldson, P.; Hartl, F.; Barone, V.; Vlček, A.; Záliš, S; The International Symposia on the Photochemistry and Photophysics of Coordination Compounds, Hong Kong (CN), 14.-19.7.2019
- Pižl, M.; Fuse, M.; Tasinato, N.; Vlček, A.; Barone, V.; Záliš, S.; Young researchers meet molecular spectroscopy, Pisa (I); 4.-5.4.2019
2018
- Guricová, M; Guldanová, R.; Tobrman, T.; Dvořák, D.; Hoskovcová, I.; 69th Annual ISE Meeting, Bologna (I); 2.-7.9. 2018
- Pižl, M.; Fuse, M.; Tasinato, N.; Barone,B.; Vlček, A.; Záliš, S.; Photoinduced Processes in Embedded Systems; Pisa (I); 24.-27.6. 2018
- Murašková, V.; Dušek, M.; Sedmidubský, D. 8th European Chemistry Congress, Paris (F), 21. – 23.6. 2018
- Záliš, S.; Pižl, M.; Fuse, M.; Barone,B.; Vlček, A.; 16th International Congress of Quantum Chemistry; Menton (F); 18.-23.6. 2018
- Pižl, M.; Záliš, S.; Seminář studetnů UFCH JH AV ČR; Prague (CZ); 12.-13.6. 2018
- Záliš, S.; Pižl, M.; Vlček, A.; Gray, H.B.; 7th JCS SYMPOSIUM; Prague (CZ); 21.-24.5. 2018
- Záliš, S.; Pižl, M.; Heyda, J.; Vlček Jr., A.; 3rd MOLIM General Meeting; Budapest (H); 19.-21.4. 2018
- Pižl, M.; Fuse, M.; Barone, V.; Antonín Vlček, A.; Záliš, S.; Anharmonicity in Medium-Sized Molecules and Clusters; Budapest (H); 16.-19.4. 2018
2017
- Záliš, S.; Heyda, J.; Pižl, M.; Vlček Jr., A.; Modeling Interactions in Biomolecules VIII; Plzeň (CZ); 3.-8.9. 2017
- Pižl, M.; Guricová, M.; Hoskovcová, I.; Záliš, S.; Modeling Interactions in Biomolecules VIII; Plzeň (CZ); 3.-8.9. 2017
- Pižl, M.; Záliš, S.; Heyda, J.; Vlček Jr., A.; 11th Triennial Congress of the World Association of Theoretical and Computational Chemists; Munich (D); 27.8.-1.9. 2017
- Pižl, M.; Vlček Jr., A.; Towrie, M.; Greetham, G.; Gray, H.B.; Záliš, S.; 22nd International Symposium on Photochemistry and Photophysics of Coordination Compounds; Oxford (UK); 9.-14.7. 2017
- Taylor, J.; Pižl, M.; Donaldson, P.; Vlček Jr., A.; Hartl, F.; 22nd International Symposium on Photochemistry and Photophysics of Coordination Compounds; Oxford (UK); 9.-14.7. 2017
- Guricová, M.; Hoskovcová, I.; Guldanová, R.; Tobrman, T.; Dvořák, D.; Ludvík, J.; 50th Heyrovsky disscusion - Molecular Electrochemistry in Organic and Organometallic Research; Třešť (CZ); 18.-22.6. 2017
- Pižl, M.; Záliš, S.; Paretzki, A.; Kaim, W; 50th Heyrovsky disscusion - Molecular Electrochemistry in Organic and Organometallic Research; Třešť (CZ); 18.-22.6. 2017
- Záliš, S.; Pižl, M.; Vlček Jr., A; Colloquium Spectroscopicum Internationale XL; Pisa (I); 11.-16.6. 2017
- Pižl, M.; Záliš, S.; Vlček Jr., A; Seminář studetnů UFCH JH AV ČR; Liblice (CZ); 9.-10.5. 2017
2016
- Záliš, S.; Pižl, M.; Heyda, J.; Vlček Jr., A.; 2nd MOLIM General Meeting; Dubrovník (HR); 10.-12.10. 2016
- Guricová, M.; Smékal, Z.; Hoskovcová, I.; 68. sjezd chemiků; Praha (CZ); 4.-7.9. 2016
- Pižl, M.; Hoskovcová, I.; Záliš, S.; 68. sjezd chemiků; Praha (CZ); 4.-7.9. 2016
- Pižl, M.; Šebera, J.; Krtil, P.; Záliš, S.; 49th Heyrovský Discussion - Electrochemical Interfaces at the Nanoscale; Třešť (CZ) 29.5.-2.6. 2016
- Guricová, M.; Hoskovcová, I.; Seminář studentů ÚFCH JH AV ČR; Liblice (CZ); 10.-11.5. 2016
- Pižl, M.; Hoskovcová, I.; Záliš, S.; Seminář studentů ÚFCH JH AV ČR; Liblice (CZ); 10.-11.5. 2016
- Pižl, M.; Hoskovcová, I.; Záliš, S.; COST Perspect CM1202 WG Meetings 1/2 and Training School; Tarragona (ES); 11.-14.4. 2016
2014
- Murašková, V.; Pižl, M.; Hoskovcová, I.; I. Pokroky anorganické chemie; Třešť (CZ); 22.-26.6. 2014
2013
- Murašková, V.; Szabó, N.; Hoskovcová I.; XXIV. International Conference on Coordination and Bioinorganic Chemistry; Smolenice (SK); 2.-7.6. 2013